


 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��� | ҩƷ | ʵ������ |
| �� | ��״ʯ��ʯ��ϡ���� | ���������������� |
| �� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
| �� | ̼���Ʒ�ĩ��ϡ���� | �����������ʷdz��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ̩������˼��ѧ���꼶��ѧ�����п��Ի�ѧ�Ծ� ���ͣ��ʴ���
(13��) ʵ���ҶԶ�����̼����ȡ�����ʵ�̽��
��1��ѡ��ҩƷ��С��������ҩƷ�������о���ʵ���¼���£�
| ��� | ҩƷ | ʵ������ |
| �� | ��״ʯ��ʯ��ϡ���� | ���������������� |
| �� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
| �� | ̼���Ʒ�ĩ��ϡ���� | �����������ʷdz��� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡΫ���о��꼶��ϰģ�⻯ѧ�Ծ����������� ���ͣ������
�����������ǻ�ѧѧϰ��һ����Ҫ������
��1��ij��ѧ�С���ԡ�����ε����ʡ�Ϊ�������̽����ѧϰ������������������⣺
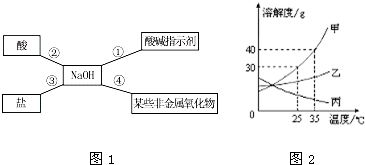
A.С��ͬѧ��ijһǷ��ũ����ȡ��һ��������Ʒ���������������ԣ��Ӿ��úͿ��з��濼�ǣ���ôҪ���������������ԣ�Ӧ��ũ����ʩ�� ���ѧʽ����
B.С��ͬѧ����ͼ�ܽ���NaOH��������ѧ���ʣ���NaOH�����������ܹ�������ѧ��Ӧ����
I.Ϊ����֤��Ӧ�٣�С�콫��ɫ��̪��Һ����NaOH��Һ�У���Һ����ɫ����� ��
II.���ݷ�Ӧ��˵��NaOH�����ܷⱣ�棬�����ڿ�����Ҫ���ʣ��仯ѧ��Ӧ����ʽΪ�� ����
III.Ϊ����֤��Ӧ���ܹ���������ѡ��������������� ��
a��Na2CO3�������� b��HCl�������� c��FeCl3 d��Ba(NO3)2
C.�������һ��ʵ���ȥFeCl����Һ��������CuCl������д����Ҫ��������ͻ�ѧ����ʽ��ʵ����Ҫ��������: �� ��
��ѧ����ʽ: ��
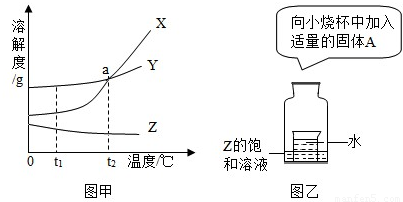
��2���ס��ҡ������ֹ������ʵ��ܽ��������ͼ��ʾ����ش�
��25��ʱ����25g������뵽50gˮ�У�����ܽⲢ�ָ���ԭ�¶Ⱥõ���Һ������Ϊ g��
��Ҫʹ35��ʱ�ӽ����͵ļ���Һ��ɸ��¶��µı�����Һ���ɲ��õ�һ�ַ����� ��
�����в������裺 a���ܽ� b������ c�����½ᾧ d������Ũ�����������к��������ң����ᴿ�IJ��������� (����ĸ���)��
�ܽ�35��ʱ���ı�����Һ���µ�25�棬������Һ�������������� ����������С�����䡱����
����100g35���ˮ�м���45g���壬����ȫ���ܽ⣬һ��ʱ������в��ּľ�������������Ϊ��ȫ���ܽ⡱��ԭ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ̩���о��꼶��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(13��) ʵ���ҶԶ�����̼����ȡ�����ʵ�̽��
��1��ѡ��ҩƷ��С��������ҩƷ�������о���ʵ���¼���£�
|
��� |
ҩƷ |
ʵ������ |
|
�� |
��״ʯ��ʯ��ϡ���� |
���������������� |
|
�� |
��״ʯ��ʯ��ϡ���� |
�����������ʻ�������ֹͣ |
|
�� |
̼���Ʒ�ĩ��ϡ���� |
�����������ʷdz��� |
����ȡ���ռ��ĽǶȷ�����һ��ѡ��ڢ���ҩƷ������ҩƷ������Ӧ�����ֱ���ʽΪ
����ѡ��ڢ���ҩƷ��ԭ���� ���Ҫ����������ɽ�����©����Ϊ____________(һ��ʵ������)��
��2��ѡ��װ�ã�ͨ������ȡ����װ�õķ�������ѡ���ù���������ȡ�����ķ���װ�ã���
��Ϊ��ѡ��������� �� ��
��3����ȡ���壮��ҩƷװ����ѡװ����ȡ���壬����____________________���ռ������������� ��
��4��������飮��ȼ�ŵ�ľ�����������У�ľ������Ϩ�������ȷ���������Ƕ�����̼��
���ļ��鷽���Ƿ���ȷ����˵�����ɣ� ��
��ȷ���������̼�ķ�����������Ӧ�����ֱ���ʽ��________________________________��
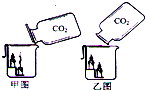
��5���������ʦ���ġ��㵹������̼������ʾʵ�飬�������ͼ�ش����⣺

�ס�����ͬѧ��ͬ�ռ�����ƿ������ͬ�Ķ�����̼���壬���ֱ�����̼�������������ձ��У�������ش�
�ټ�ͬѧ�ü�ͼ���㵹��ʽ���ձ����㵹�㹻�Ķ�����̼�����ǹ۲쵽�������������¶���Ϩ������������ǣ�Ϊʲô�����¶���Ϩ��
__________________________________________________��
����ͬѧ����ͼ���㵹��ʽ���ձ����㵹�㹻�Ķ�����̼�����ǹ۲쵽�Ľ������������û��Ϩ��������ϵѧ��֪ʶ ����������ͼ���㵹��ʽΪʲô������̼û�н����ձ���
__________________________________________________��
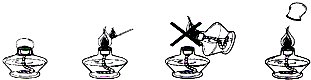
��6�������̲��С��ƾ��Ƶ�ʹ�÷����������۲�����ͼ�λش����⣺

��ֻ����ȼ�ŵĻ���ϸľ��ȥ��ȼ�ƾ��ƣ��þƾ�����ȼ��һֻ�ƾ��ƻ�����ʧ��ԭ����_______________________________________________��
��Ϊ��������ʧ������ƾ��ƺ���������ƾ��������Ӿƾ���Ӧ���õ�ñ������ʹ�ƾ���___________________________________�������Ӿƾ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com