
| ��� | l | 2 | 3 | 4 |
| ��ˮ������(g) | 10 | 10 | 10 | 10 |
| ʣ����������(g) | 7.25 | 4.05 | 3 | m |
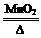 2KCl+3O2�� (1��)
2KCl+3O2�� (1��)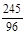 =
=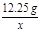 x=4.8g ��2�֣�
x=4.8g ��2�֣�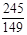 =
=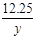 y=7.45g ��2�֣�
y=7.45g ��2�֣�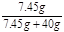 ��100%=15.7% ��2�֣�
��100%=15.7% ��2�֣�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
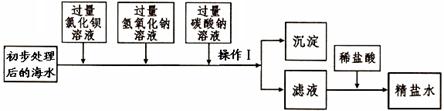
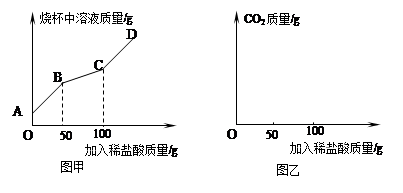

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1�s71 | B��2�s71 | C��1�s79 | D��2�s79 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
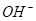 �������йأ�һ���������Һ��
�������йأ�һ���������Һ��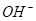 ����Խ�࣬����Һ�ļ���Խǿ��ͬ�����10%��NaOH��Һ��10%��KOH��Һ����Һ�ܶ���ͬ����ȣ����Ը�ǿ����
����Խ�࣬����Һ�ļ���Խǿ��ͬ�����10%��NaOH��Һ��10%��KOH��Һ����Һ�ܶ���ͬ����ȣ����Ը�ǿ����| A��NaOH | B��KOH | C��һ��ǿ | D�����Ƚ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com