
(10��)��������۵ļ۸����ܴ�����ۡ��ָж�û�����Բ��죬��������ۺ���������۵ijɷ��кβ�ͬ�أ�ʵ��С��ͬѧչ��̽����
���������ϡ�
�����������ֱ���ɱ��ƶ�����������ӹ����ɣ�����һ�����İ����ᡢ̼��Ƶȡ�
������������ɡ����Ƿۡ��ӹ����ɣ����Ǽӹ������л��õ��������ƣ����ǵ���Ҫ�ɷ���̼��ơ�
��������г�̼����⣬�����ɷ־����������ᷴӦ���ɶ�����̼��
�ܰ���������ܽ���ˮ������Ũ���Ტ���Ȼ���ֻ�ɫ���ɫ��
��̼�����Ũ�����ܷ�����Ӧ�����ɶ�����̼��
���Ȼ�����Һ�����ԡ�
��������롿��������ۺ���������۵�����������ڣ�
����������ۺ��� ��
����������۲��������
��̼��ƺ�����ͬ��
��ʵ��̽����
| ʵ �� �� �� | �� �� | �� �� |
| ��1���ֱ�������������ۺ���������۷����Թ��У�������ˮ������һ��ʱ��μ� �� | ��������۵��ϲ���Һ��죬��������۵���Һû�б�ɫ | ����ٳ��� |
| ��2���ֱ�������������ۺ���������۷����Թ��У�������ˮ�����ˣ� �����ȡ� | ��������۵���Һ�л�ɫ���֣��ֲ���ڣ���������۵���Һû���������� | ���� ���� |
| | ��������� | ��������� |
| ����۵����� | 100g | 100g |
| ������������� | 460.0g | 500g |
| �ձ����������ʵ������� | 520.0g | 558.2g |
��������롿NaOH
��ʵ��̽������1����ɫ��̪��Һ ��2������Һ�м���Ũ���� ��
��3����CaCO3 +2HCl=CaCl2 +H2O +CO2�� �����У���Ϊ����������л���NaOH������Ҳ�������ᷴӦ �� 95
���������������������롿������Ŀ��������Ϣ��֪������������ۺ���NaOH
��ʵ��̽������1������NaOH��һ�ּ��ˮ��Һ��ʹ��ɫ��̪��Һ��죬��Ϊʵ����۲���ٳ����������Ǽ�����ɫ��̪
��2���������Ϣܰ���������ܽ���ˮ������Ũ���Ტ���Ȼ���ֻ�ɫ���ɫ�����Էֱ�������������ۺ���������۷����Թ��У�������ˮ�����ˣ�����Һ�м���Ũ���ᣬ���ȣ��۲쵽��������۵���Һ�л�ɫ���֣��ֲ���ڣ���������۵���Һû�������������Բ���ڳ���
��3��������۵���Ҫ�ɷ���̼��ƣ��������ᷴӦ����ʽΪ��CaCO3 +2HCl=CaCl2 +H2O +CO2����С����˼·�����У���Ϊ����Ϊ����������л���NaOH������Ҳ�������ᷴӦ
�ڸ��������غ㶨�ɣ���ѧ��Ӧǰ����������䣬���Ը�������������������̼������=100g+500g-558.2g=41.8g���ٸ��ݷ�Ӧ����ʽΪ��CaCO3 +2HCl=CaCl2 +H2O +CO2����CO2��CaCO3��������ϵ�����CaCO3������
�⣺��CaCO3������Ϊx
CaCO3 +2HCl=CaCl2 +H2O +CO2��
100 44
X 41.8g
100��44=x��41.8g
X=95g
̼��Ƶ���������=95g/100g��100%=95%
���㣺CaCO3��NaOH�Ļ�ѧ���ʣ����ݷ�Ӧ����ʽ����


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʯұ��������һ�����ӵĹ��̡�������ԭ��������һ����̼���������ķ�Ӧ���û�ѧ����ʽΪ ��
��1��ij������ÿ��������5000t��������76%�ij�����ʯ���ó������Ͽ����ղ�����98%�������������Ƕ��٣�
����д��������̣������ȷ��0.1t��
��2��ij��ѧ��ȤС����ʵ������ģ������ԭ����ʵ�飬���Թ������ijɷֽ���̽����
�����ʵ�顿��һ����̼��ԭ��������������ĩ��ʵ��װ����ͼ���ƾ���b�������� ��
������ʵ�顿��С�鰴����ʵ����ƣ���һ���¶��½�����ʵ�飬����������ݣ�
�ٷ�Ӧǰ�����ܺ���ʢ��������ĩ��������66.0g�������ܵ�����Ϊ60.0g����
�ڷ�Ӧ�����ܺ���ʢ��ɫ�����������m����ȴ�����³�������
�����������ۡ���ʵ���У��������ڹ����ĩ�ɺ�ɫȫ����Ϊ��ɫ��С��ͬѧ��Ϊ�ú�ɫ����ȫ����������С��ͬѧ��������ɣ����Ǵ������������ϡ�
���������ϡ�
��һ����̼��ԭ�������Ĺ������ijɷ��뷴Ӧ�¶ȡ���Ӧʱ��������йء�
��һ����̼��ԭ��������ʵ������й�������������������������������������
��
| ���� ���� | ���������� | �������� | ������ | ���� |
| ��ɫ | ��ɫ | ��ɫ | ��ɫ | ��ɫ |
| �ܷ������� | �� | ���� | ���� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ijƷ�ƴ����к�������NaCl����ѧ��ȤС���ͬѧ����������ʵ��̽������ȡ12g��Ʒ�����ձ��У�����ϡ���������ٲ�������Ϊֹ�������Ƴ�����ϡ�����������ų����������Ĺ�ϵ��ͼ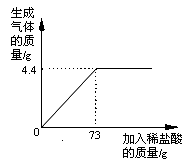
��1������ǡ����ȫ��Ӧʱ������CO2������Ϊ g
��2���������Ʒ�к����ʵ����������Ƕ��٣�������������һλС������ͬ��
��3�����㵱�����봿��ǡ����ȫ��Ӧʱ��������Һ���������������Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��6�֣���ȡNaCl��BaCl2�Ĺ�������32.5g������100g����ˮ����ȫ�ܽ����û����Һ����μ�����������Ϊ10%��Na2SO4��Һ����Ӧ����BaSO4�������������������Na2SO4��Һ��������ϵ��ͼ��ʾ���Իش��������⣺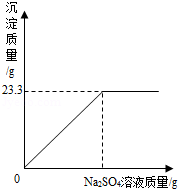
��1����ȫ��Ӧ������BaSO4������ ��g��
��2��ǡ����ȫ��Ӧʱ����Na2SO4��Һ�������Ƕ��ٿˣ�
��3��ǡ����ȫ��Ӧʱ������Һ�����ʵ����������Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
Ϊ�ⶨijBaCl2��Һ������������������ȡ100gBaCl2��Һ�����ϼ���ϡH2SO4����Ӧ��������Һ���������ϡH2SO4��������ϵ��ͼ��ʾ������Ӧ����ʽ��BaCl2+H2SO4=BaSO4��+2HCl����ش�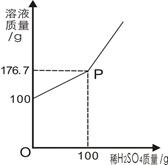
��1��P������_______________��
��2����Ӧ����BaSO4����������Ϊ_________g��
��3����ԭBaCl2��Һ����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��7�֣���һ�ձ���ʢ��60.2gBaCO3��BaCl2�ķ�ĩ״���������м���188.8gˮʹ������п�������ȫ�ܽ⣬Ȼ����������μ���������������Ϊ10%��ϡ������146gʱǡ�÷�Ӧ��ȫ�����������ش����⣺
��1���ڵμ���������й۲쵽������ʵ�������� ��
��2��������μ���140gʱ���ձ�����Һ�ﺬ�����ʵ��� ����д��ѧʽ��
��3������ǡ�÷�Ӧ��ȫʱ�ձ������ò�������Һ���������������ȷ��0.1g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
(10��)ij��ȤС��ֱ���80.00 g 10%��NaOH��Һ�м��������ͬ������������ͬ�����ᣬ��ַ�Ӧ��������Һ����������ֱ��������ȫ������û���κα仯������ϸ����ɡ��������ظ��������ɡ�������ֱ���ƵõĹ����������ٷ����仯��ʵ���ã���80.00 g10%��NaOH��Һ�м���80.00 mL����ʱ����Һǡ�ó����ԣ�������õIJ������������±���ʾ��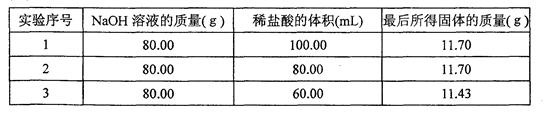
��1����������������Һʱ��Ҫ�ò��������Ͻ��裬��Ŀ���ǣ� ��
��2����80.00 g 10%NaOH��Һ�м�����������Ϊ100.00 mLʱ��������ù����������Ϊ11.70 g����ԭ���ǣ� ��
��3����ʽ�����80.00 g 10%NaOH��Һ�м���40.00 mL����ʱ��������ù�����NaCl������������С���������λ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��7�֣��кͷ�Ӧ����ѧ��ѧ����Ҫ��ѧϰ���ݣ������ճ������ũҵ�������й㷺��Ӧ�á�
��1����ͼ��ʾ���������������Һ������Ӧ��������Һ��pH�ı仯���ߡ��������ͼ�л�ȡ��Ϣ���ش��������⣺
��ͼ1ͼ���ʾ���������������Һ������Ӧ��������Һ��pH�仯�����и÷�Ӧ��ʵ������ǰ���ͼ2�е� ������ң�ͼ��ʾ���еġ�
��������M���ʾ ��
�����ձ����㵹20g��������Ϊ4.00��������������Һ������3�η�̪��Һ��������ε�����������Ϊ3.65����ϡ���ᣬ�ߵα���ֱ����Һ�պñ�Ϊ ɫΪֹ������ȥϡ����20g����Ӧ����Һ��������������Ϊ (�����ȷ��0.1��)��
��2��Ϊ֤���кͷ�Ӧ�Ƿ��ȷ�Ӧ��ijС���������ͼ��
ʾ��ʵ�������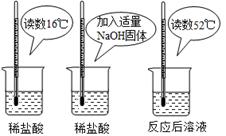
������ͼʵ�飬��ͬѧ��Ϊ��NaOH��ϡ���ᷢ�����кͷ�Ӧ�Ƿ��ȷ�Ӧ����ͬѧ��Ϊ����ͬѧ�ó�
������۵����ݲ���ѧ�������� ��
��3��Ϊ̽��Ӱ���кͷ�Ӧ�ų��������ٵ����أ������ֽ���������ʵ�飺�ڱ��ΪA��B��C��
D��E����ֻ�ձ��и�װ��36.5g ������������Ϊ5%��10%��15%��20%��25%�����ᣬ
����������ֻ�ձ��зֱ����40g20% ������������Һ�����������¶ȣ����ݼ�¼���£�
| �ձ���� | A | B | C | D | E |
| ����������������� | 5% | 10% | 15% | 20% | 25% |
| ��Ӧ����Һ�¶ȣ��棩 | 24�� | 34�� | 46�� | 54�� | 54�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��3�֣���(Ti)���ѺϽ���21���͵���Ҫ�������ϡ��ѿ�ͨ�����·�Ӧ�Ƶã�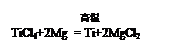
���㣺Ҫ�Ƶ�96g�ѣ���Ҫþ��������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com