
ijƷ�ƴ����к�������NaCl����ѧ��ȤС���ͬѧ����������ʵ��̽������ȡ12g��Ʒ�����ձ��У�����ϡ���������ٲ�������Ϊֹ�������Ƴ�����ϡ�����������ų����������Ĺ�ϵ��ͼ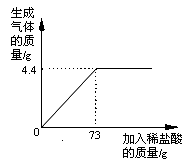
��1������ǡ����ȫ��Ӧʱ������CO2������Ϊ g
��2���������Ʒ�к����ʵ����������Ƕ��٣�������������һλС������ͬ��
��3�����㵱�����봿��ǡ����ȫ��Ӧʱ��������Һ���������������Ƕ��٣�
��1��4.4g��2��11.7% ��3��16.3%
���������������1��������Ʒ�е���Ҫ�ɷ�Ϊ̼���ƣ��������ᷴӦ���ɶ�����̼���塣����ͼʾ��֪��������73gϡ����ʱ����������������ﵽ�����ֵ������ʱ̼����ǡ����ȫ��Ӧ������ǡ����ȫ��Ӧʱ���ɶ�����̼������Ϊ4.4g��
��2�����������֪����֪��Ϊ������̼��������δ֪��Ϊ��Ʒ�к����ʵ���������������˼·Ϊ���ɸ��ݷ�Ӧ�ж�����̼��̼���Ƶ�������ϵ���̼���Ƶ���������һ���������Ʒ�к��Ȼ��Ƶ�����������
��3�����������֪����֪��Ϊ������̼��������δ֪��Ϊ������Һ�����ʵ���������������˼·Ϊ��������ҺΪ�Ȼ�����Һ���ɸ��ݷ�Ӧ�ж�����̼���Ȼ��Ƶ�������ϵ��������Ȼ��Ƶ��������ټ�����Ʒ��ԭ���Ȼ��Ƶ���������Ϊ������Һ�е����ʵ��������ٸ��������غ㶨�ɿ����������Һ�����������ɼ����������Һ�����ʵ���������������������£�
��2���⣺����Ʒ�к�̼���Ƶ�����Ϊx����Ӧ�����Ȼ��Ƶ�����Ϊy
Na2CO3+2HCl==2NaCl+H2O+CO2��
106 117 44
x y 4.4g
106��44=x��4.4g
x=10.6g
117��44=y��4.4g
y=11.7g
��Ʒ�к����ʵ���������Ϊ��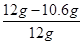 ��100%��11.7%
��100%��11.7%
��3��������Һ�����ʵ���������Ϊ��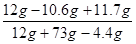 ��100%��16.3%
��100%��16.3%
�𣺣�1�����ɶ�����̼������Ϊ4.4g��
��2����Ʒ�к����ʵ���������Ϊ11.7%��
��3��������Һ�����ʵ���������Ϊ16.3%��
���㣺�ۺϼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��12gij�������90gˮ�г���ܽ����ˣ��˳��ù���2g�������Һ����������������
| A��10% | B��11.7% | C��12% | D��13.3% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ij����ҩ���ı�ǩ��Ҫ������ͼ��ʾ���ֲⶨ�ø�Ƭ�����Ƿ���ϱ�ע��������ʵ�飺ȡ10Ƭ�ø�Ƭ���������ྻ���ձ��У������ձ��м���50gijŨ��ϡ���ᣬǡ����ȫ��Ӧ����Ƭ�������ɷֲ�����ˮ��Ҳ����ϡ���ᷴӦ����Ӧ�в���������ȫ���ų�������Ӧ������ձ���ʣ�����ʵ�����Ϊ64.5g��
��1����Ӧ�������� g������̼���˷�Ӧ�Ļ�����Ӧ����Ϊ�� ����
��2��ͨ������˵��ʵ�ʸƺ����Ƿ����ע�����
��3���Լ�������ϡ�������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��7�֣���ҵ��ͨ���û�������Ҫ�ɷ�FeS2��Ϊԭ���������ᣬ�Ƚ���������飬Ȼ�������������Ʊ����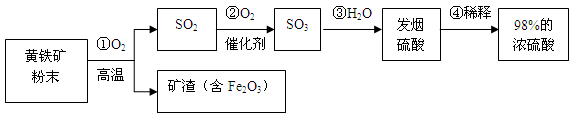
�� �Ƚ�������ʯ�����Ŀ���� ��
�� д��ϡ��Ũ����ľ���������� ��
�� ��10g98%��Ũ����ϡ�ͳ�9.8%��ϡ���ᣬ��Ҫ��ˮ g��
�� ȡ20g������Ʒ����Fe2O3���������м���300g������������Ϊ9.8%��ϡ���ᣨ��֪�����е�Fe2O3��ϡ����ǡ����ȫ��Ӧ�õ�Fe2(SO4)3��Һ������ͨ����ѧ����ʽ����ÿ�����Ʒ��Fe2O3������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
С����ʵ���������31��6g���������ȡ��������ȫ��Ӧ��ʣ���������Ϊ28��4g����ʣ������ܽ⡢���ˡ�����ϣ����ն������̡�����㣺
��1����������������Ϊ g��
��2�����ն������̵�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��7�֣�ijͬѧ��������ʵ�飺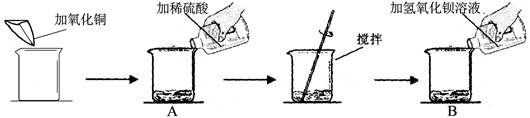
ʵ�����ݼ�����ʵ���������±���
| | ��һ��[��Դ:ѧ���ƣ���Z��X��X��K] | �ڶ��� |
| ������ͭ��������g�� | m | m |
| ��ϡ�����������g�� | 50 | 100 |
| ������������Һ��������g�� | 100 | 100 |
| B����Ҫ���� | ����ɫ���� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��12�֣���ͼ��ijͬѧ��ɡ�ʵ��5 һ�������������Ȼ�����Һ�����ơ��С�����
50 g��������Ϊ6%���Ȼ�����Һ����ȫ���̡�
��1��ͼ�еĴ����У�
�� ��
�� ��
��2���������Ƶ���ˮ��NaCl ����������С��6%��������������ԭ���У����پ�
�����㣩��
�� ��
�� ��
��3����Ũ��Һ����ϡ��Һʱ������������� ��Ҫ��50 g��������Ϊ98%��Ũ����ϡ��Ϊ��������Ϊ20%�����ᣬ��Ҫˮ�������� ����ʵ������Ũ��������ϡ�������Ҫ�����У����㡢 �����ȡ���ȴ������װƿ�����ϱ�ǩ��
��4��ȡijϡ������Ʒ10g����5%��NaOH��Һ��μ��뵽��Ʒ�У��ӱ߽��衣��ҺpH�ı仯��ͼ��ʾ���Իش�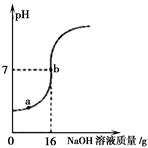
��a����Һ�к��е������� ��
�ڵ�pH=7ʱ������NaOH��Һ��NaOH������Ϊ g��
�ۼ���ϡ���������������������д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
(10��)��������۵ļ۸����ܴ�����ۡ��ָж�û�����Բ��죬��������ۺ���������۵ijɷ��кβ�ͬ�أ�ʵ��С��ͬѧչ��̽����
���������ϡ�
�����������ֱ���ɱ��ƶ�����������ӹ����ɣ�����һ�����İ����ᡢ̼��Ƶȡ�
������������ɡ����Ƿۡ��ӹ����ɣ����Ǽӹ������л��õ��������ƣ����ǵ���Ҫ�ɷ���̼��ơ�
��������г�̼����⣬�����ɷ־����������ᷴӦ���ɶ�����̼��
�ܰ���������ܽ���ˮ������Ũ���Ტ���Ȼ���ֻ�ɫ���ɫ��
��̼�����Ũ�����ܷ�����Ӧ�����ɶ�����̼��
���Ȼ�����Һ�����ԡ�
��������롿��������ۺ���������۵�����������ڣ�
����������ۺ��� ��
����������۲��������
��̼��ƺ�����ͬ��
��ʵ��̽����
| ʵ �� �� �� | �� �� | �� �� |
| ��1���ֱ�������������ۺ���������۷����Թ��У�������ˮ������һ��ʱ��μ� �� | ��������۵��ϲ���Һ��죬��������۵���Һû�б�ɫ | ����ٳ��� |
| ��2���ֱ�������������ۺ���������۷����Թ��У�������ˮ�����ˣ� �����ȡ� | ��������۵���Һ�л�ɫ���֣��ֲ���ڣ���������۵���Һû���������� | ���� ���� |
| | ��������� | ��������� |
| ����۵����� | 100g | 100g |
| ������������� | 460.0g | 500g |
| �ձ����������ʵ������� | 520.0g | 558.2g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��8�֣�
ij��ѧ��ȤС��ʹ����ͼ��ʾװ�ã���ij��пͭ�Ͻ�ijɷֽ��в�������ȡ����ϡ�������ձ��У��������м���15��0g�Ͻ���Ʒ��ʼ��ʱ������������ƽ�Ķ�����¼���±��У�������м��㣺
��1����Ӧ��ȫ����������������Ƕ��٣�
��2��пͭ�Ͻ���ͭ�������Ƕ��٣�
��3����Ӧ��ȫ����Һ�����ʵ����������Ƕ��٣�
| | ���ձ� | ��������� | ����Ͻ�� 5���� | ����Ͻ�� 10���� | ����Ͻ�� 30���� |
| ������g�� | 21��3 | 169��7 | 184��6 | 184��3 | 184��3 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com