
Ϊ�˲ⶨij��ĩ״��ͭ��ͭ��п�Ͻ���Ʒ��ͭ����������������ͬѧȡһ�������Ļ�ͭ��Ʒ�����ձ��У���ȡ40gϡ������Ĵμ����ձ��У�����ַ�Ӧ��ʵ���������£�
|
|
��һ�� |
�ڶ��� |
������ |
���Ĵ� |
|
����ϡ����������g�� |
10 |
10 |
10 |
10 |
|
ʣ������������g�� |
9.10 |
8.45 |
7.80 |
7.80 |
��1��������Ӧ�Ļ�ѧ����ʽΪ ��
��2������п�������г����ڶ�����������������x���ı���ʽ
��
��3������ϡ���������ʵ���������Ϊ���٣�
��4����36.5%��Ũ��������40g����ϡ���ᣬ��ҪŨ���������Ϊ���٣�
��5����ͭ��Ʒ��ͭ����������Ϊ���٣�
��1��Zn��2HCl��ZnCl2��H2��
��2��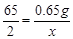 [��65:2��(9.10g��8.45g):x]
[��65:2��(9.10g��8.45g):x]
��3��7.3%
��4��8g
��5��80%
����������1��п�ڽ����˳�����λ����֮ǰ�ܰ������е����û���������Ӧ�Ļ�ѧ����ʽΪZn+2HCl�TZnCl2+H2����
��2���ڶ��μ���10g���ᣬ���¹�����9.10g�����8.45g��������Ҳ����8.45g���7.80g��˵��ÿ����10g�����ܹ�ʹ�������0.65g�����Եڶ��μ�����������п������=9.10g-8.45g=0.65g�����У�
Zn+2HCl�TZnCl2+H2��
65 2
0.65g x
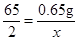
��3����10gϡ������HCl������Ϊy
Zn+2HCl�TZnCl2+H2��
65 73
0.65g y
 ���y=0.73g
���y=0.73g
����ϡ���������ʵ���������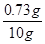 ��100%=7.3%
��100%=7.3%
��4����ҪŨ���������=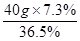 =8g
=8g
��5���ɵڶ���ʵ���֪����һ�μ���10gϡ����ʱ����Ӧ����п������Ϊ0.65g����ԭ��ͭ��Ʒ������=9.10g+0.65g=9.75g���ɵ��Ĵ�ʵ���֪����Ʒ��ͭ������7.80g
��ͭ��Ʒ��ͭ����������=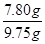 ��100%=80%
��100%=80%


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��һ�� | �ڶ��� | ������ | ���Ĵ� | |
| ����ϡ����������g�� | 10 | 10 | 10 | 10 |
| ʣ������������g�� | 9.10 | 8.45 | 7.80 | 7.80 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��һ�� | �ڶ��� | �ڶ��� | ���Ĵ� | |
| ����ϡ����������g�� | 10g | 10g | 10g | 10g |
| ʣ������������g�� | 8.7g | 7.4g | 6.1g | 5.45g |
| 65 |
| 2 |
| 4.55g |
| X |
| 65 |
| 2 |
| 4.55g |
| X |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��һ�� | �ڶ��� | ������ | ���Ĵ� | |
| ����ϡ����������g�� | 10 | 10 | 10 | 10 |
| ʣ������������g�� | 9.10 | 8.45 | 7.80 | 7.80 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����� | 1 | 2 | 3 | 4 |
| ϡ��������� | 10g | 10g | 10g | 10g |
| ʣ���������� | 8.7g | 7.4g | 6.7g | 6.7g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��һ�� | �ڶ��� | ������ | ���Ĵ� | |
| ����ϡ����������g�� | 10 | 10 | 10 | 10 |
| ʣ������������g�� | 9.10 | 8.45 | 7.80 | 7.80 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com