
����Ŀ��ij��ɫ������ܺ���CH4��CO��H2�е�һ�ֻ��֣����ν�������ʵ��(����ÿһ����Ӧ�����վ���ȫ)��
�ٽ���ɫ������ȼ�գ���ȼ�պ����ɵ�����ͨ��Ũ�����װ�ã�װ�õ���������7.2g�����ٽ�ʣ������ͨ��ʢ��Һ��װ�ã�װ�õ���������8.8g�������ƶϴ������
A. ԭ��ɫ�����п���ֻ����CH4��H2 B. ԭ��ɫ�����п���ֻ����CH4
C. ԭ��ɫ�����п���ֻ����CO��H2 D. ԭ��ɫ������һ������̼Ԫ�ء���Ԫ��
���𰸡�A
���������������⣬Ũ��������ȼ�����ɵ�ˮ������������Һ��������ȼ�����ɵĶ�����̼��Ũ��������������������Һ�����������ӣ�˵��ȼ�ռ�������ˮ�������˶�����̼����Ͽ�ȼ����ȫȼ�յĹ��ɣ����жϻ�������к�C��HԪ�أ�������ˮ��������̼��������һ��ȷ�����������C��HԪ�ص������ȣ������жϻ�����������о�����������
�⣺�����ɵ�����ͨ��Ũ���ᣬװ����������7.2g�����ж�ȼ��������7.2gˮ��˵����������к���Ԫ�أ�����Ԫ������=7.2g��![]() ��100%=0.8g��
��100%=0.8g��
������ͨ������������Һ��װ����������8.8g�����ж�ȼ��������8.8g������̼��˵����������к�̼Ԫ�أ���̼Ԫ������=8.8g��![]() ��100%=2.4g��
��100%=2.4g��
����������C��HԪ��������=12����1��4��=3��1������������C��HԪ��������=2.4g��0.8g=3��1����˿��жϻ���������ֻ�м��飻Ҳ����ֻ����CO��H2�������ܺ��м���������κ�һ��������
A��ԭ��ɫ�����п���ֻ����CH4��H2���Ǵ���ģ�
B��ԭ��ɫ�����п���ֻ����CH4������ȷ�ģ�
C��ԭ��ɫ�����п���ֻ����CO��H2������ȷ�ģ�
D��ԭ��ɫ������һ������̼Ԫ�ء���Ԫ�أ�����ȷ����
��ѡA��


 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������������ͼ��ʾװ���Ʊ��������壬��ش��й����⣺
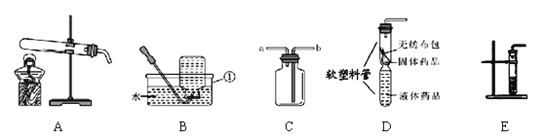
(1)�����ٵ�����Ϊ__________��
(2)����Aװ����ȡ�������÷�Ӧ�Ļ�ѧ����ʽΪ_________________________��
(3)ѡ��Bװ���ռ�������������������ԭ����___________________(дһ�ּ���)��
(4)��װ��C�ռ�����������ʱ�������ǵ�ľ������_______(����a������b��)����
(5)װ��D��E����������ȡ������̼��װ��D���װ��E����Ҫ�ŵ�����______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ͼ��ʾ��ʵ��װ��ͼ��ա�
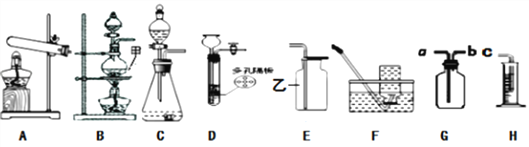
(1)Bװ���м���������Ϊ______��Eװ�����ҵ���������Ϊ___________��
(2)ʵ������Cװ����ȡ����ʱ��������Ӧ�Ļ�ѧ����ʽΪ___________��
(3)���д���ʯ��̼���Ʒ�ĩ��ϡ���ᡢϡ���ᣬѡ����ʵ�������Dװ����ȡCO2���壬�仯ѧ����ʽΪ______________�����ڷ�Ӧ�����У�ʹ��Ӧֹͣ�IJ�����_________���ռ�������̼һ����װ��___________��
(4)С��ͬѧ��п����ϡ������ȡ������С����ΪС����ȡ�����������������������ˮ���������_______(�ѧʽ)��ʹ��_________(������)��Һ��ȥ������װ��G��H�ռ����ⶨ�������������Gװ�ø�װ�ķ�����_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס��ҡ������ֹ������ʵ��ܽ��������ͼ��ʾ��
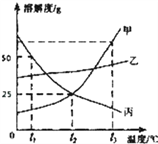
��1������ t2��ʱ���ܽ��Ϊ____��
��2��t1��ʱ 30g ���� 50g ˮ����ܽ��γ�____������͡������͡�����Һ��
��3���ı�����Һ�к����������ҿ��Բ���____ �ᴿ�ף�
��4������˵���������____������ţ���
A���� t3��ס��ҡ����ı�����Һ���µ� t2��ʱ���������������Ǽ�
B���� t2��50g ���ı�����Һ��ˮϡ�ͳ�������������Ϊ 10%�ı���Һ����Ҫ�õ��������У��� ��������������Ͳ��50ml������ͷ�ι�
C��t2���ҵı�����Һ�м������ף��ҵ����ʵ�������������
D��t3��������ס��ҡ����ı�����Һ�У����ܼ������DZ�
E�����ӽ����͵ļ���Һ��ɼı�����Һ�����������������ܲ��䣮
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͨ���Ի�ѧ��ѧϰ��ͬѧ����������ʵ������ȡ������йع��ɣ�������˼���ʵ��װ�ý����о���������ͼ1�ش����⣺
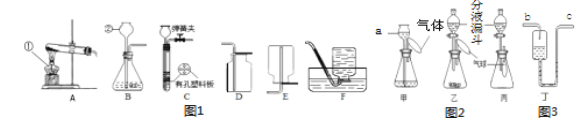
��1��д���������ֵ��������ƣ���________
��2��ͬѧ�����ø��������ȡһƿ�ϴ�������������Ӧѡ������װ���е�A��____����A-F��ţ�������װ����С����ΪAװ���л�ȱ��_______���÷�Ӧ�Ļ�ѧ����ʽΪ_____________��
��3��ʵ������ȡ�������� C װ�ã����п����ϰ��Ϸ��õ�ҩƷ��_____����װ�õ��ŵ���_______��
��4������ͼ2��ͬѧ����Ƶļס��ҡ���һ�顰������ʵ�飬��ش�
��װ���У���a�м���һ����ˮ��ῴ��_____________��һ��ʱ���ڹ۲쵽�����С���仯��˵����װ�����������á����������õ���װ���У�����ƿ��װ��NaOH���壬����ƿ�����Һ©���е�ˮ���������ʹ����Ҫԭ����________________�����������õı�װ���У�����ƿ��ʢ��CO2����ʹ�����ʹ����Һ©���е�Һ�������_______��
A.ϡ������Һ B.NaOH��Һ C.NaCl��Һ D. NH4NO3��Һ
����ͼ3װ�ö���ͬѧ������ҽ����Һ�۲��Һ�����ĵκ�����������ϴ��װ�ã���ش�����Ӧ��_________���b����c�������롣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��С��ͬѧ������������������ij��Ԫ�صĻ��ϼۻ����˳��л�ѧ�̲��г�����һЩ���ʣ��������˲������ʼ��ת����ϵ����ͼ������ͼ��ʾ(ͼ����������ʾ���ʼ��ת��)����֪A��B��C��D����һ����ͬ��Ԫ�أ�E��F��D����������ͬ��Ԫ�أ�E��H��Ӧ����F�ҷų��������ȡ�
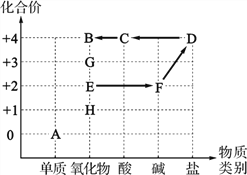
��ش��������⣺
(1)C�Ļ�ѧʽΪ__________��
(2)E¶���ڿ�����һ��ʱ������������ӣ���ԭ�������_____________________��
(3)1.2 g A��2.0 g O2ǡ����ȫ��Ӧ�����ɵIJ�����__________________(�ѧʽ)��
(4)��G����Ԫ�ص���������Ϊ30%����G����һ��Ԫ�ص����ԭ������Ϊ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��ȤС���ͬѧһ��̽�������Ļ�ѧ���ʡ�
������ʵ����
�Ѵ�ĥ�����˿��������ͭ��Һ�У���˿�����к�ɫ�����������ó����ۣ�����ͭ�Ľ������˳����___________����д����˿��������ͭ��Һ�еķ�Ӧ����ʽ____________��
��������⣩
С��ͬѧ��֤��ʵ��ʱ�������쳣�����е��Թ�����˿����û�к�ɫ������֣����������˺�ɫ���塣Ϊ��̽��������С��ͬѧ����������ʵ�飺
ʵ����� | �� | �� | �� |
ʵ�� |
|
|
|
�۲�ʱ��(����) | 3 | 3 | 3 |
���ɹ�����ɫ | ��ɫ | ��ɫ | ��ɫ |
����������ۣ�
(1)ͨ������ʵ���ܵó���˿�����к�ɫ������������¶��йص�ʵ�������_________��
(2)ͨ������ʵ������ܵó���˿�����к�ɫ����������¶��йأ����ܵó���_________�йء�
����˼�����ۣ�
��Դ��쳣�����㻹��̽����������_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʩ���ڵĺܶྰ�㶼�Ѿ���ȫ��������������ʢ�����硰��������������ʩ��Ͽ�ȡ���;���γ���Щ����춴�ĸ���ԭ���뵱�ص�ʯ��ʯ�йء�ij��ѧ�ġ���ѧ��ȤС�顱Ϊ�˲ⶨ������������ʯ��ʯ��Ʒ��CaCO3�ĺ�����ȡ8g��Ʒ���������ձ��У��ټ���100gϡ����ǡ����ȫ��Ӧ���ձ���ʣ������105.8�ˣ�������Ʒ�����ʲ���Ҳ����Ӧ��������ˮ��������CO2���ܽ�����أ���������ٷֺ�ǰһλС�������Լ��㣺
��1����Ʒ��CaCO3�����������Ƕ���_________��
��2��������Һ�����ʵ����������Ƕ���_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ƽ��仯�����������������о��й㷺��Ӧ��
(һ)�Ƽ��仯�����Ӧ��
(1)������ȱ���������ļ�����________��
(2)��������Ҳ����һ�����ĺ������ʣ��ֽ������������γɸֽ�����������������в��漰�IJ�����_________�� a�������� b���ϳɲ��� c�����ϲ���
(3)�����Ϳ�ͼ���ԭ����������ʯ�Һ�ˮ��Ӧ���ȣ��÷���ʽ��ʾ�䷴Ӧ__________��
(��)��������(CaO2)������
(1)CaO2����Ԫ�صĻ��ϼ�Ϊ_____________��
(2)CaO2����ϡ���ᷢ�����ֽⷴӦ����Ӧ�Ļ�ѧ����ʽΪ_____________��
(3)���;���������������Ҫ�ɷ�ΪCaO2������ˮ������Ӧ����O2��������һ�ּ�仯ѧʽΪ_______��Na2O2Ҳ����ˮ��Ӧ��ԭ����CaO2��ͬ����ȴ������Ϊ��Ϻ����Ĺ���������������ܵ�ԭ��___________________��
(��)�������ƾ�����Ʊ�
�����ϣ��������ƾ���(CaO2��yH2O)������Ϊ��ɫ���������ᣬ�����ھƾ���
�Ʊ�ԭ����CaCl2��H2O2��NH3��H2O ![]() CaO2��yH2O����NH4Cl��װ�����¡�
CaO2��yH2O����NH4Cl��װ�����¡�

(1)װ��A���Թ��ڷ�����Ӧ�Ļ�ѧ����ʽΪ______________________��
(2)װ��C���ñ�ˮԡ�����¶���0�����ң����ܵ�ԭ����Ҫ�У�
��. �÷�Ӧ�Ƿ��ȷ�Ӧ���¶ȵ����������CaO2��yH2O���ʣ�
��. _____________________________��
(3)��Ӧ���������ˡ�ϴ�ӡ����º�ɿɻ��CaO2��yH2O��
��ϴ��ʱ����95%�ľƾ���Һϴ�ӵ��ŵ���_________________��
�ڼ��龧����ϴ�Ӹɾ��ķ���Ϊ________________________��
(��)�������ƾ�����ɵIJⶨ
��ȡ21.6�˾������ȷ����Ƕ�������ȷֽ�ʵ�飬�����Ƴɹ����������¶ȹ�ϵͼ(�������ƾ�������ʱ����ʧȥ�ᾧˮ)

(1)0��150�������������ı��ԭ����______________________��
(2)������ͼ��֪y=________��
(3)350��ʱ������Ӧ�Ļ�ѧ����ʽΪ_____��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com