
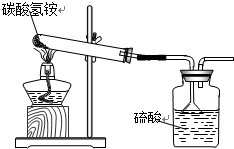
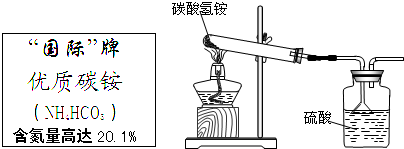
 �ݹ��ұ���̼炙�����NH4HCO3�������õ���95%��ijͬѧ������ͼװ�ú����·�Ӧ����������ʵ�飨�������ʲ�������Ӧ����
�ݹ��ұ���̼炙�����NH4HCO3�������õ���95%��ijͬѧ������ͼװ�ú����·�Ӧ����������ʵ�飨�������ʲ�������Ӧ���� NH3��+H2O��+CO2��
NH3��+H2O��+CO2��| ʵ����� | 1 | 2 | 3 | ƽ��ֵ |
| ʹ����Ʒ������/g | 8.00 | 8.00 | 8.00 | 8.00 |
| ������Һ���ӵ�����/g | 1.71 | 1.69 | 1.70 | 1.70 |
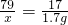
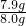 ��100%=98.75%��
��100%=98.75%��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
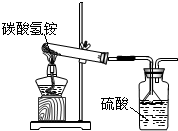 �ݹ��ұ���̼炙��ʵ�NH4HCO3�������õ�����95%���º�ͬѧ����ͼװ�ú����·�Ӧ����������ʵ�飬�Ҽ������ʲ�������Ӧ��NH4HCO3=NH3��+H2O+CO2����
�ݹ��ұ���̼炙��ʵ�NH4HCO3�������õ�����95%���º�ͬѧ����ͼװ�ú����·�Ӧ����������ʵ�飬�Ҽ������ʲ�������Ӧ��NH4HCO3=NH3��+H2O+CO2����| ʵ����� | 1 | 2 | 3 | ƽ��ֵ |
| ʹ����Ʒ���������ˣ� | 8.00 | 8.00 | 8.00 | 8.00 |
| ������Һ���ӵ��������ˣ� | 1.71 | 1.69 | 1.70 | 1.70 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ʵ����� | 1 | 2 | 3 | ƽ��ֵ |
| ʹ����Ʒ���������ˣ� | 8.00 | 8.00 | 8.00 | 8.00 |
| ������Һ���ӵ��������ˣ� | 1.71 | 1.69 | 1.70 | 1.70 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
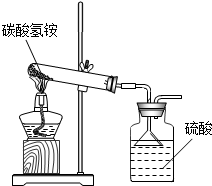 ��2013?����ģ�⣩�ݹ��ұ���̼炙�����NH4HCO3�������õ���95%��ijͬѧ������ͼװ�ú����·�Ӧ����������ʵ�飨�������ʲ�������Ӧ����
��2013?����ģ�⣩�ݹ��ұ���̼炙�����NH4HCO3�������õ���95%��ijͬѧ������ͼװ�ú����·�Ӧ����������ʵ�飨�������ʲ�������Ӧ����
| ||
| ʵ����� | 1 | 2 | 3 | ƽ��ֵ |
| ʹ����Ʒ������/g | 8.00 | 8.00 | 8.00 | 8.00 |
| ������Һ���ӵ�����/g | 1.71 | 1.69 | 1.70 | 1.70 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
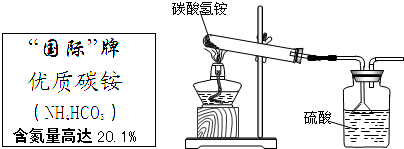
 NH3��+H2O+CO2�������� NH3+H2SO4�T��NH4��2SO4
NH3��+H2O+CO2�������� NH3+H2SO4�T��NH4��2SO4| ʵ����� | 1 | 2 | 3 | ƽ��ֵ |
| ʹ����Ʒ���������ˣ� | 8.00 | 8.00 | 8.00 | 8.00 |
| ������Һ���ӵ��������ˣ� | 1.71 | 1.69 | 1.70 | 1.70 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
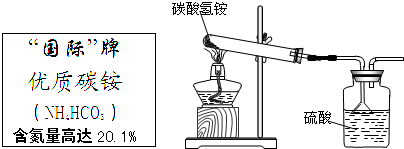
| ||
| ʵ����� | 1 | 2 | 3 | ƽ��ֵ |
| ʹ����Ʒ���������ˣ� | 8.00 | 8.00 | 8.00 | 8.00 |
| ������Һ���ӵ��������ˣ� | 1.71 | 1.69 | 1.70 | 1.70 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com