
ʵ��֤������ͬ��ͬѹ�£���ͬ������κ������к��еķ�������ͬ���ݴ˻ش���������
(�����������ָͬ��ͬѹ��)��(1)
��ͬ�����CO(ú������Ҫ�ɷ�)�� (ʯ��Һ��������Ҫ�ɷ�)��ϣ����û�������У�C��H��OԪ�ص���������________��
(ʯ��Һ��������Ҫ�ɷ�)��ϣ����û�������У�C��H��OԪ�ص���������________��
(2)
ʯ��Һ������ú��ȼ�յ���Ҫ��ӦΪ��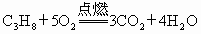 ��
�� ��ȼ�����������塢�����ܡ�����ܡ����ӿ��صȲ�����ɣ�Ŀǰ�ܵ�ú�����û�Ѹ�����ӣ��������Ϸ�Ӧ������ʹ��ʯ��Һ�������û�����ʹ�ùܵ�ú�������¹��õ������ԭ���������ȹ����ϵ����������________��ԭ����________��
��ȼ�����������塢�����ܡ�����ܡ����ӿ��صȲ�����ɣ�Ŀǰ�ܵ�ú�����û�Ѹ�����ӣ��������Ϸ�Ӧ������ʹ��ʯ��Һ�������û�����ʹ�ùܵ�ú�������¹��õ������ԭ���������ȹ����ϵ����������________��ԭ����________��
(3)
���ʯ�͡�ú��Դ�������ٵ�������������ڻ�����������Դ������Ϊ21������Դ�㷺��ȼ�շ��ȶࡢ����Ⱦ��ȼ�ϵ������ȡ������________��|
���� (1)6��1��2��(2)�¹���߽����ֱ����ԭ���С����ͬ�����COȼ������ �� �� �١�(3) �١�(3)
����������������Ϣ��ͬ����� CO�� ��ϣ����Ӹ���Ӧ��ͬ����ȡ��С������1��1���з���C��H��O������Ϊ4��8��1����Ԫ��������6��1��2��������Ӧ���� ��ϣ����Ӹ���Ӧ��ͬ����ȡ��С������1��1���з���C��H��O������Ϊ4��8��1����Ԫ��������6��1��2��������Ӧ���� ���Ӹ�������ú��ȼ������ ���Ӹ�������ú��ȼ������ �ǵ������Һ��ʯ����ȼ������ �ǵ������Һ��ʯ����ȼ������ �����1/10������������¹���Ҫ�Ľ������ֱ��������С����ʯȼ��ȼ�ղ������� �����1/10������������¹���Ҫ�Ľ������ֱ��������С����ʯȼ��ȼ�ղ������� ���Ӽ�����Ⱦ�Ƕȣ����ȼ��Ϊ ���Ӽ�����Ⱦ�Ƕȣ����ȼ��Ϊ �������ȡ������̫���ֽܷ�ˮ�� �������ȡ������̫���ֽܷ�ˮ��
���������ԡ�ȼ��Ӧ�á���һ�����Ϊ���У�����ͬѧ�Ƿ�������ͽ������������� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com