
�Ҵ��׳ƾƾ���������ҽ��������Ҳ����ȼ�ϡ�����ȫȼ�յĻ�ѧ����ʽ�ɱ�ʾΪ��C2H6O+3O2 2 CO2+ 3 H2O ��
2 CO2+ 3 H2O ��
23g�Ҵ���ȫȼ�������Ķ��ٿ�������
�Ҵ�����ȫȼ�ջ����һ����̼��ijʵ���÷�Ӧǰ������ʵ��������±���
| ���� | �Ҵ� | ���� | ������̼ | ˮ | һ����̼ |
| ��Ӧǰ������g�� | 4.6 | 8.8 | 0 | 0 | 0 |
| ��Ӧ��������g�� | 0 | 0 | 6.6 | 5.4 | a |
 _____ CO2+ _____ H2O + _____ CO ��
_____ CO2+ _____ H2O + _____ CO ��  ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ʵ��������һƿ�����ռ��������˿����еĶ�����̼�����ֱ��ʡ�Ϊ�ⶨ��ƿ�ռ�Ĵ��ȣ���ȡ����Ʒ20g������ˮ�����Һ����������μ����Ȼ�����Һ��������ȫ����Ӧ���������ɳ����������������Ȼ�����Һ������ϵ��ͼ��ʾ��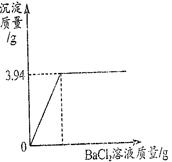
��1����ͼ�п��Կ�������Ӧ�����ij�������� g��
��2�����ռ���Ʒ��̼���Ƶ������Ƕ���g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�����ѧ����У���������Ҫ90L�����������ѧ����С����п�����ʵ���������Ϊ20%��ϡ������ȡ��Щ���壮��֪�������ܶ�Ϊ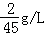 ������
������
��1���������ʵ���������Ϊ20%��ϡ���������Ϊ���٣�
��2��������п�루1��������������ϡ�����ַ�Ӧ��������Һ�����ʵ���������Ϊ���٣��������ȷ��0.1%��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ij��ȤС��ӷ������ײ���һ����Ƭ����������20%��ϡ�����У����������������������������������ͼ��������������Ĥ��Ӧʱû��H2�������������ʲ����ᷴӦ������ش�
��1����ͼ�п������÷�ӳ������H2_______g��
��2�����������Ļ�ѧ����ʽΪ��__________����Ƭ�������ʵ�����Ϊ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��6.8 g�����ʵ�п�������뵽ʢ��50.0 g������ϡ������ձ��У����ʲ�����ˮ��Ҳ����ϡ���ᷴӦ������ַ�Ӧ�Ƶ��ձ������ʵ�������Ϊ56.6 g���Լ��㣺
��1������������������
��2��п��������п��������������ȷ��0.1%����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���Ϣ������
(6��)�����ǵ���Ҫ�ɷ���̼���(�����ɷֲ�����ˮҲ�����ᷴӦ)����ѧ��ȤС��Ϊ�˲� ����������̼��Ƶĺ�����������ʵ�飺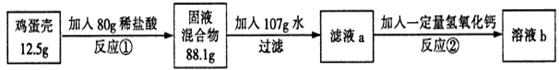
��Ӧ�ٽ�������������պ÷�Ӧ��һ�룬��Ӧ��ǡ����ȫ��Ӧ����ش��������⣺
��1����Ӧ�ٵĻ�ѧ����ʽΪ___________________________��
��2��������֪�����г����̼�������(X)�ı���ʽ______________��
��3���ü�������̼��Ƶ���������Ϊ_______��
��4�������������Ƶ�����Ϊ_______��
��5����Һb�����ʵ���������Ϊ_______��
��6����36.5����Ũ��������80g����ϡ�������ˮ������Ϊ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�������綯��һ��ʹ��Ǧ�����ء�ijǦ������ʹ�õ�����Һ����������Ϊ20%��ϡ���ᡣ��ش������й����⣺
��1������100g��������Ϊ98%��Ũ���ᣨ�ܶ�Ϊ1.84g/cm3�����Ƹ�ϡ����ʱ����Ҫ����ˮ���ܶ�Ϊ1g/cm3�������Ϊ mL����ȷ��0.1����
��2����ʵ�����Ҫ�����м��㡢��ȡ��ϡ�����ơ�װƿ������ǩ��
����д�²�ı�ǩ��
��3������Ͳ��ȡˮʱ���Ӷ�����������Һ������������______ 20�����>������<����=������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪20��ʱ����ص��ܽ��Ϊ31.6g���ڸ��¶��½�20g����ط���50gˮ�У���ֽ��裬��������Һ�����ʵ���������ԼΪ
| A��24.0% | B��28.6�� | C��31.6�� | D��40.0�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ݻ�ѧ����ʽ���㣺��ȫ�ֽ�340g������������Ϊ10%�Ĺ���������Һ�еĹ������⣨H2O2����������������������
| A��16g | B��160g | C��32g | D��1.6g |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com