
 ×100%���������Ʒ��Na2CO3������������
×100%���������Ʒ��Na2CO3������������ ×100%�������ǡ����ȫ��Ӧ�����ɵ���Һ�����ʵ�����������
×100%�������ǡ����ȫ��Ӧ�����ɵ���Һ�����ʵ����������� =
= ��
�� =
= ��
�� ×100%=39.8%��
×100%=39.8%�� =
= ��
�� ×100%=10.2%��
×100%=10.2%��

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
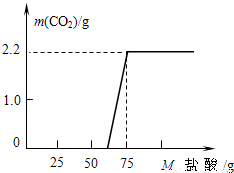
 ijͬѧ��ȡ13.3g ��NaOH��Ʒ������ΪNa2CO3������ˮ�����Һ100g��Ȼ������Һ����μ���ϡ���ᣬ��������CO2�������ⶨNa2CO3��������ʵ���ü���ϡ��������������CO2�����������ϵ��ͼ��ʾ��
ijͬѧ��ȡ13.3g ��NaOH��Ʒ������ΪNa2CO3������ˮ�����Һ100g��Ȼ������Һ����μ���ϡ���ᣬ��������CO2�������ⶨNa2CO3��������ʵ���ü���ϡ��������������CO2�����������ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
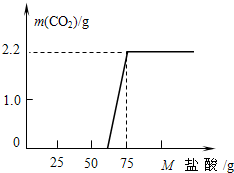
 ijУ�о���ѧϰС���ͬѧ̽��ʵ���Ҿ��õ�NaOH�ı��ʳ̶ȣ���ȡ13.3g��NaOH��Ʒ������ΪNa2CO3���������Һ��Ȼ������Һ����μ�����������Ϊ14.6%ϡ���ᣬ����ϡ����������Ķ�����̼�����������ϵ��ͼ��
ijУ�о���ѧϰС���ͬѧ̽��ʵ���Ҿ��õ�NaOH�ı��ʳ̶ȣ���ȡ13.3g��NaOH��Ʒ������ΪNa2CO3���������Һ��Ȼ������Һ����μ�����������Ϊ14.6%ϡ���ᣬ����ϡ����������Ķ�����̼�����������ϵ��ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ijͬѧ��ȡ13.3g ��NaOH��Ʒ������ΪNa2CO3������ˮ�����Һ100g��Ȼ������Һ����μ���ϡ���ᣬ��������CO2�������ⶨNa2CO3��������ʵ���ü���ϡ��������������CO2�����������ϵ��ͼ��ʾ��
ijͬѧ��ȡ13.3g ��NaOH��Ʒ������ΪNa2CO3������ˮ�����Һ100g��Ȼ������Һ����μ���ϡ���ᣬ��������CO2�������ⶨNa2CO3��������ʵ���ü���ϡ��������������CO2�����������ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ijУ�о���ѧϰС���ͬѧ̽��ʵ���Ҿ��õ�NaOH�ı��ʳ̶ȣ���ȡ13.3g��NaOH��Ʒ������ΪNa2CO3���������Һ��Ȼ������Һ����μ�����������Ϊ14.6%ϡ���ᣬ����ϡ����������Ķ�����̼�����������ϵ��ͼ��
ijУ�о���ѧϰС���ͬѧ̽��ʵ���Ҿ��õ�NaOH�ı��ʳ̶ȣ���ȡ13.3g��NaOH��Ʒ������ΪNa2CO3���������Һ��Ȼ������Һ����μ�����������Ϊ14.6%ϡ���ᣬ����ϡ����������Ķ�����̼�����������ϵ��ͼ���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com