
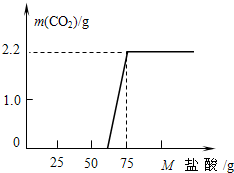
 ijͬѧ��ȡ13.3g ��NaOH��Ʒ������ΪNa2CO3������ˮ�����Һ100g��Ȼ������Һ����μ���ϡ���ᣬ��������CO2�������ⶨNa2CO3��������ʵ���ü���ϡ��������������CO2�����������ϵ��ͼ��ʾ��
ijͬѧ��ȡ13.3g ��NaOH��Ʒ������ΪNa2CO3������ˮ�����Һ100g��Ȼ������Һ����μ���ϡ���ᣬ��������CO2�������ⶨNa2CO3��������ʵ���ü���ϡ��������������CO2�����������ϵ��ͼ��ʾ��| ̼���Ƶ����� |
| ��Ʒ������ |
| �������� |
| ��Һ���� |
| 106 |
| x |
| 44 |
| 2.2g |
| 117 |
| y |
| 44 |
| 2.2g |
| 5.3g |
| 13.3g |
| 40 |
| 8g |
| 58.5 |
| z |
| 17.55g |
| 172.8g |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijУ�о���ѧϰС���ͬѧ̽��ʵ���Ҿ��õ�NaOH�ı��ʳ̶ȣ���ȡ13.3g��NaOH��Ʒ������ΪNa2CO3���������Һ��Ȼ������Һ����μ�����������Ϊ14.6%ϡ���ᣬ����ϡ����������Ķ�����̼�����������ϵ��ͼ��
ijУ�о���ѧϰС���ͬѧ̽��ʵ���Ҿ��õ�NaOH�ı��ʳ̶ȣ���ȡ13.3g��NaOH��Ʒ������ΪNa2CO3���������Һ��Ȼ������Һ����μ�����������Ϊ14.6%ϡ���ᣬ����ϡ����������Ķ�����̼�����������ϵ��ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
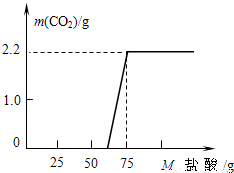
 ijͬѧ��ȡ13.3g ��NaOH��Ʒ������ΪNa2CO3������ˮ�����Һ100g��Ȼ������Һ����μ���ϡ���ᣬ��������CO2�������ⶨNa2CO3��������ʵ���ü���ϡ��������������CO2�����������ϵ��ͼ��ʾ��
ijͬѧ��ȡ13.3g ��NaOH��Ʒ������ΪNa2CO3������ˮ�����Һ100g��Ȼ������Һ����μ���ϡ���ᣬ��������CO2�������ⶨNa2CO3��������ʵ���ü���ϡ��������������CO2�����������ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ijУ�о���ѧϰС���ͬѧ̽��ʵ���Ҿ��õ�NaOH�ı��ʳ̶ȣ���ȡ13.3g��NaOH��Ʒ������ΪNa2CO3���������Һ��Ȼ������Һ����μ�����������Ϊ14.6%ϡ���ᣬ����ϡ����������Ķ�����̼�����������ϵ��ͼ��
ijУ�о���ѧϰС���ͬѧ̽��ʵ���Ҿ��õ�NaOH�ı��ʳ̶ȣ���ȡ13.3g��NaOH��Ʒ������ΪNa2CO3���������Һ��Ȼ������Һ����μ�����������Ϊ14.6%ϡ���ᣬ����ϡ����������Ķ�����̼�����������ϵ��ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ��������У�����п���ѧģ���Ծ���5�·ݣ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com