
| ||
| ||
| ||
| 56x+16y |
| 3.6g |
| 44y |
| 2.2g |
| x |
| y |
| 1 |
| 1 |
| 56x |
| 16y |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������������������ÿ��Ҫ����ʳ��5g���� |
| B��ҽ��������ˮ��0.5%���Ȼ�����Һ |
| C���������еĶ�����̼����������ﵽ1%ʱ����������к� |
| D��ͨ����ʳ����Լ��3%��5%�Ĵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CaCl2��Na2CO3 |
| B��Na2SO4��Ba��OH��2 |
| C��FeCl2��Na2SO4 |
| D��BaCl2��NaOH |
�鿴�𰸺ͽ���>>
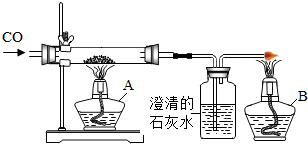
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
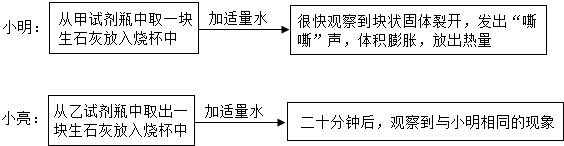
| ʵ�鲽�� | ʵ������ | ʵ����� | |
| С�� | �����Լ�ƿ��ȡ��һ����ʯ�����������ձ��У���������ˮ��Ȼ��μӷ�̪��Һ | ��̪��Һ��� | �����������ƣ�˵����Ʒ�Ѳ��ֱ��� |
| С�� | �����Լ�ƿ��ȡ��һ����ʯ�ң����������ձ��У���������ϡ���� | �����ݲ��� | ����̼��ƣ�˵����Ʒ�Ѳ��ֱ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com