
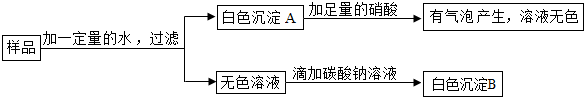
���� ��Ʒ�м���ˮ���ɵð�ɫ����A����ɫ��Һ����ɫ����A�м����������ݲ�����˵����̼���Σ���ɫ��Һ�μ�̼������Һ���а�ɫ����B��˵�����������ƣ�
��� �⣺��1����Ʒ�м���ˮ���ɵð�ɫ����A����ɫ��Һ����ɫ����A�м����������ݲ�����˵����̼���Σ���ɫ��Һ�μ�̼������Һ���а�ɫ����B��˵�����������ƣ���ѡ��D��
��2�����������ܣ���ɫ����A������̼��ƺ��������ƵĻ���̼�������������Ʒ�Ӧ����̼��ƺ��������ƣ�����ʽΪCa��OH��2+Na2CO3�TCaCO3��+2NaOH��
�ʴ�Ϊ����1��D��2������ȷ���п�����̼��ƺ��������ƵĻ�����Ϊ������������ˮ
Ca��OH��2+Na2CO3�TCaCO3��+2NaOH
���� ���⿼���˳������ʵı��ʳɷֵ�̽���ͻ�ѧ����ʽ����д����ɴ��⣬�����������ʵ����ʽ��У�


 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | N2 | B�� | O2 | C�� | NO | D�� | NO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Һ�����ʵ�����������С | B�� | A�ܽ�ȼ�С | ||
| C�� | ���ʵ��������� | D�� | ��Һ���ܶ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | BaCO3��HCl��Һ | B�� | BaCl2��Һ��K2SO4��Һ | ||
| C�� | CO2��KOH��Һ | D�� | Na2SO4��Һ��KNO3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
���� | ���� | ƻ��֭ | ���� | ¯������ |
| pH | 2.5 | 3.1 | 8.5 | 12.4 |
| A�� | θ���������ٳ�ƻ�� | B�� | pH��ֽ�ɾ�ȷ�������Һ���pH | ||
| C�� | ������ʹ��ɫʯ����Һ��� | D�� | ¯�������ļ��Ա�����ǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
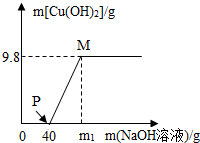 ��CuCl2��HCl��100g�����Һ�У���μ���������������Ϊ40%NaOH��Һ���μӷ�Ӧ��NaOH��Һ���������ɳ���������ϵ��ͼ����ͼ�ش�
��CuCl2��HCl��100g�����Һ�У���μ���������������Ϊ40%NaOH��Һ���μӷ�Ӧ��NaOH��Һ���������ɳ���������ϵ��ͼ����ͼ�ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com