
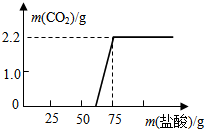
 ij��ѧ��ȤС��Ϊ�˲ⶨʵ�����о��õ�NaOH�ı��ʳ̶ȣ�����������ʵ�飬�ȳ�ȡ13.3g��NaOH��Ʒ������ΪNa2CO3���������Һ��Ȼ������Һ����μ�����������Ϊ14.6%��ϡ���ᣬ��������CO2�������ⶨNa2CO3���������Ӷ���һ��ȷ����Ʒ��NaOH�ı��ʳ̶ȣ�ʵ���ü���ϡ��������������CO2�����������ϵ��ͼ��ʾ����������Ա�ʵ���˵���д�����ǣ�������
ij��ѧ��ȤС��Ϊ�˲ⶨʵ�����о��õ�NaOH�ı��ʳ̶ȣ�����������ʵ�飬�ȳ�ȡ13.3g��NaOH��Ʒ������ΪNa2CO3���������Һ��Ȼ������Һ����μ�����������Ϊ14.6%��ϡ���ᣬ��������CO2�������ⶨNa2CO3���������Ӷ���һ��ȷ����Ʒ��NaOH�ı��ʳ̶ȣ�ʵ���ü���ϡ��������������CO2�����������ϵ��ͼ��ʾ����������Ա�ʵ���˵���д�����ǣ�������| A�� | ����CO2���������Ϊ2.2g | B�� | Na2CO3������Ϊ5.3g | ||
| C�� | ���ʵ�NaOH������Ϊ4.0g | D�� | NaOH�ı��ʳ̶�Ϊ30% |
���� �������������ܺͿ����еĶ�����̼��Ӧ����̼���ƺ�ˮ���ܺ�ϡ���ᷴӦ�����Ȼ��ƺ�ˮ��̼���ƺ�ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼�����ö�����̼���������̼���Ƶ��������������Ƶ��������ʵ���������NaOH��Ӧ���õ��������ʵ�������̼���Ƶ�����������ʵ��������Ƶ����������������Ʒ��NaOH�ı��ʳ̶ȼ��ɣ�
��� �⣺��ͼ����Ϣ��֪������������̼��������2.2g��
��̼���Ƶ�����Ϊx��
Na2CO3+2HCl�T2NaCl+H2O+CO2����
106 44
x 2.2g
$\frac{106}{x}$=$\frac{44}{2.2g}$
x=5.3g
����������Ʒ�Ӧ���Ȼ�������Ϊy��
���Ȼ��ⷴӦ��������������Ϊ��13.3g-5.3g=8g��
NaOH+HCl=NaCl+H2O��
40 36.5
8g y
$\frac{40}{8g}$=$\frac{36.5}{y}$
y=7.3g��
����ʵ�������������Ϊz��
2NaOH+CO2�TNa2CO3+H2O��
80 106
z 5.3g
$\frac{80}{z}$=$\frac{106}{5.3g}$
z=4g
��NaOH�ı��ʳ̶�Ϊ��$\frac{4g}{13.3g-5.3g+4g}$��100%=33.3%��
ͨ���Ƶ���֪��
A������CO2���������Ϊ2.2g����A��ȷ��
B��Na2CO3������Ϊ5.3g����B��ȷ��
C�����ʵ�NaOH������Ϊ4.0g����C��ȷ��
D����NaOH�ı��ʳ̶�Ϊ33.3%����D����
��ѡ��D��
���� ������Ҫ����ѧ�����ü��跨�ͻ�ѧ����ʽ���м�����ƶϵ�������ͬʱ�����˷���ͼ�����ݵ�����������ʱҪע��淶�Ժ�ȷ�ԣ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ˮ����ͭ��ĩ��ˮ���� | B�� | Ũ����¶�ÿ����к��������� | ||
| C�� | ��ʯ��¶�ÿ������������� | D�� | ������̼��������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
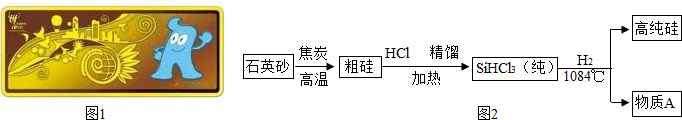
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 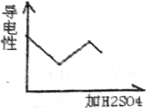 | B�� |  | C�� | 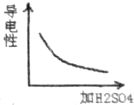 | D�� | 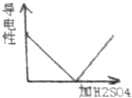 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������� | B�� | ������� | C�� | �ֽ����� | D�� | �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
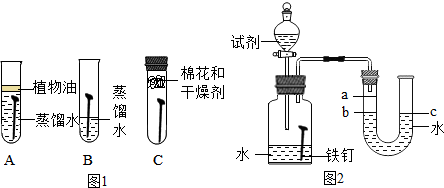
| Ӱ������ | ʵ����� | ʵ������ | ʵ����ۼ����� |
| ���¶� | װ�â����25����»����У�װ�â����40����»����� | ����װ������������ʴ����U�ι���Һ�����b��������a����������ʱ��� | ��ʴ�����ʢ�������ʴ�����뷴Ӧʱ���¶��й��¶�Խ�ߣ�������ʴ�ٶ�Խ�� |
| ���������� | ����ͬ�¶��£�װ�â���ƿ�м�����������10mL�Ҵ� | ����װ������������ʴ����U�ι���Һ�����b��������a����������ʱ��� | ������ʴ�����������ĺ����йأ������ĺ���Խ�࣬������ʴԽ�� |
| ��ˮ��Һ�ĵ����� | ����ͬ�¶��£�װ�â���ƿ�м���10mL�Ȼ�����Һ��װ�â���ƿ�м���10mL�Ҵ� | ����װ������������ʴ����U�ι���Һ�����b��������a����������ʱ��� | ������ʴ������ˮ��Һ�ĵ������йأ����ܵ������Һ�У���ʴ�����ʼӿ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
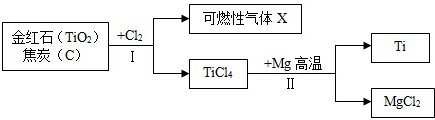
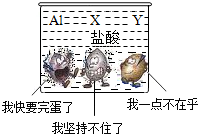
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com