��2011?������ģ�⣩2011������3?15�����ع⣺�ӱ�ʡ������ʯ��ׯ�ȵ���ijЩ��ҵΥ�����������涨���û��յ�������ֽ�����������о��ռ��ӫ�������������������ʲ������ͽ�ֽ��������ѧ�ռ�֪ʶ�ش��������⣺
��1�����ռ���������Ƶ��׳ƣ���ͨ���ֱ���Ϊ
��������
��������
��
��2���ɷ��й���˵�����վ���ָ������
��ʴ
��ʴ
��ǿ����ѧʵ������Ҫ��ȡһ���������������ƣ�Ӧ����
�ڢ�
�ڢ�
��
��ֽƬ�ϡ����ñ������ס��С�ձ��С�����ƽ���̡�����ƽ����
��3���������ƹ����к�ǿ����ˮ�ԣ�����ʯ�Ұ�������Ϻ��Ϊ��ʯ�ң��dz��õ�����������������ѧ����֪ʶ��д����ʯ����ˮ��Ӧ�Ļ�ѧ����ʽ��
CaO+H2O�TCa��OH��2
CaO+H2O�TCa��OH��2
���������岻���ü�ʯ�Ҹ������
�٢�
�٢�
��
��CO
2 ��CO ��H
2 ��O
2 ��SO
2��4����ȤС��ͬѧΪ��̽��ʵ�����о��õ��������ƹ���ijɷ֣��������й�ʵ�飮����������һ���������̽�����
[�Թ������]
�����ȫ����NaOH��
�����ȫ����Na
2CO
3��
�������NaOH��Na
2CO
3����
[ʵ�����]
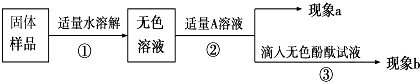
[������]
��������aΪ�����ݲ�����������A��Һ��
ϡ����
ϡ����
��˵�����������Ѿ����ʣ������ݲ����ķ�Ӧ�Ļ�ѧ����ʽ��
Na2CO3+2HCl�T2NaCl+H2O+CO2��
Na2CO3+2HCl�T2NaCl+H2O+CO2��
��
����A��Ca��OH��
2��Һ������a�а�ɫ����������bΪ��ɫ��̪��Һ���ɫ�����ɫ����Ϊ
CaCO3
CaCO3
���ѧʽ������ʵ��
����
����
����ܡ����ܡ���˵����Ʒ����NaOH��
����A��CaCl
2��Һ����ʵ������aΪ
�а�ɫ��������
�а�ɫ��������
����������bΪ
��ɫ��̪��Һ����ɫ
��ɫ��̪��Һ����ɫ
�������������
[��˼]���õ��������Ʊ��ʵ�ԭ���ǣ��û�ѧ����ʽ��ʾ��
2NaOH+CO2�TNa2CO3+H2O
2NaOH+CO2�TNa2CO3+H2O
��





 ��2011?������ģ�⣩��ͼ��ʾ��һ�������·�����ij��ѧ��Ӧ��������˵����ȷ���ǣ�������
��2011?������ģ�⣩��ͼ��ʾ��һ�������·�����ij��ѧ��Ӧ��������˵����ȷ���ǣ�������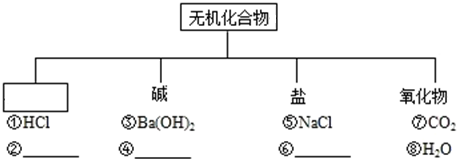
 ��2011?������ģ�⣩��ͼΪA��B��C����Ԫ�ص����ӽṹʾ��ͼ���ش��������⣺
��2011?������ģ�⣩��ͼΪA��B��C����Ԫ�ص����ӽṹʾ��ͼ���ش��������⣺