
��ͼ�ǡ���������Ƭ��Ʒ��ǩͼ��
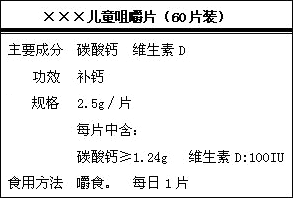
| ��������ͯ��Ƭ ��60Ƭװ�� |
| [��Ҫ�ɷ�]̼��ƣ�ά����D [���]2.5g/Ƭ��ÿƬ�к�̼��ơ�1.24g��ά����D 100l.U. [ʳ�÷���]��ʳ,ÿ��һƬ [��Ч]���� |
���ݱ�ǩ������Ϣ������ش���������: �������ȷ��0.1��
��1�� ��Ҫ�ɷ�̼����и�Ԫ�ص���������Ϊ______________��ÿƬ�����ٺ���Ԫ�ص�����Ϊ______________________g��
��2�� С��ͬѧΪ�ⶨ��̼��Ƶĺ�����ע�Ƿ���ʵ����ȡ��4Ƭ��Ƭ�����������ձ��У���μ���ϡ���ᣬ�����ٷų�����Ϊֹ������ȥϡ����40.0g�������ձ���ʣ����Ϊ47.8g�������ձ��������ٶ���Ƭ�����ɷֲ���ϡ���ᷴӦ����
�Լ��㣺�����ɶ�����̼������____________��
��ϡ���������ʵ���������____________��
��ͨ�������жϸ�Ƭ��̼��Ƶĺ�����ע�Ƿ���ʵ________________��
 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д� ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��������ͯ��Ƭ ��60Ƭװ�� |
| [��Ҫ�ɷ�]̼��ƣ�ά����D [���]2.5g/Ƭ��ÿƬ�к�̼��ơ�1.24g��ά����D 100l��U�� [ʳ�÷���]��ʳ��ÿ��һƬ [��Ч]���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��������ͯ��Ƭ��60Ƭװ�� |
| [��Ҫ�ɷ�]̼��ƣ�ά����D [���]2.5g/Ƭ��ÿƬ�к�̼��ơ�1.24g��ά����D 100l��U�� [ʳ�÷���]��ʳ��ÿ��һƬ [��Ч]���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��������ͯ��Ƭ ��60Ƭװ�� |
| [��Ҫ�ɷ�]̼��ƣ�ά����D [���]2.5g/Ƭ��ÿƬ�к�̼��ơ�1.24g��ά����D 100l��U�� [ʳ�÷���]��ʳ��ÿ��һƬ [��Ч]���� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com