
������(��Ҫ�ɷ���CaCO3)�ӹ��ɵġ�����ۡ�����һ���������ҩ�ġ�����Ʒ���ɽ������г��ϳ�����һЩ��ð�ġ�����ۡ����������۹۲��ѱ���٣�Ϊ�����������ṩ���������ҳ���١�����ۡ���������ѧ�����ϵIJ��죮����չ��̽����(ֻ��д�����롢����������Ҫ˵������ʵʩ�ľ�����)
����1����١�����ۡ�����ζ���ܲ�ͬ��
����1��ȡ�����ֱ���һ�����ǵ���ζ���ֱ���ζ�����죮
����2��________________��
����2��________________��
����3��________________��
����3��________________��
|
��������2����١�����ۡ���ˮ�е��ܽ��Կ��ܲ�ͬ ��������2��ȡ�����ֱ��������ˮ�����裬�۲����ǵ��ܽ��������� ��������3����١�����ۡ����ᷴӦ��������ܲ�ͬ ��������3��ȡ�����ֱ��������ϡ���ᣬ�۲����������� ��������4����١�����ۡ����պ�����в�ͬ������ ��������4��ȡ�����ֱ��ھƾ��ƻ���������Ƭ�̣��۲����ǵ�ɫ̬�仯(������ֻҪ��������) |


 ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д� ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д� ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2013?��ͨһģ������۳�Ϊ������ϲ����Ȼ��һЩ���������ñ��Ƿ�ð����������ۣ�һ�о���ѧϰС��Ϊ�˼���ij����۵���٣�����������̽����
��2013?��ͨһģ������۳�Ϊ������ϲ����Ȼ��һЩ���������ñ��Ƿ�ð����������ۣ�һ�о���ѧϰС��Ϊ�˼���ij����۵���٣�����������̽�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�꽭��ʡ�Ͼ��й�¥���п���ģ��ѧ�Ծ����������� ���ͣ�̽����
ij�о���ѧϰС���ij����۽���������̽����
���������ϡ�������Ļ�ѧ�ɷ���Ҫ�ǣ�̼��Ƽ�����̼��þ�Ϳǽǵ���(�����ʵ�һ��)��
�ڿǽǵ��ײ�����ˮ������Ũ������Ⱥ����ɫ��
�ۼ��������ʵ���ǡ����Ƿۡ�����Ҫ�ɷ־���̼��ơ�
���������ۡ����������������ǽǵ������������������ ���� ���л����������������
�ڼ�������е�̼��ƺ���Ҫ��������۴�
��ʵ��̽�����о���ѧϰС���������������������
����һ ��������۵����
ȡ�����������Ʒ���Թ��У� �����۲쵽 ������������������ۡ�
������ �����������е�̼��ƺ���
������������������ͼ��ʾʵ��װ�á�ijѧ����סC�����ܣ���A��װ������ˮ��ȡ���ϲ����ӣ�����������A��ˮ����ȫ�����£�����Ϊ��װ���Ƿ�©���� ��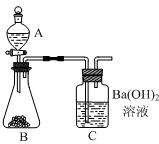
�����ȷ��ȡ6.000 g�������Ʒװ������B�У���A��װ��ϡ���ᡣ
�������B���������Ʒ�еμ�������ϡ���ᡣ������B��C�пɹ۲쵽������Ϊ �� ��
���������ȫ��Ӧ��C�е���Һ�� �� ������Ƶð�ɫ���������Ϊ11.820g�����������Ʒ��̼��Ƶ���������Ϊ100%��
��ʵ�鷴˼����ô˲ⶨ��������ܵ�ԭ���� ������������ţ�
����Ʒ�к���̼��þ
������δ�μ�����
��CO2�����ٶ�̫�쵼��δ��Ba(OH)2��ȫ����
��װ��B��ˮ������HCl�Ƚ���װ��C��
����չ���졿��ͬѧ����ڷ������в���Ҫװ��C��ֻҪ�õ�����ƽȷ����װ��A��B��Ӧǰ����������Ϳ��Եõ��������Ʒ��̼��Ƶĺ���������Ϊ�Ƿ���� ����˵������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�꽭��ʡ�Ͼ��й�¥���п���ģ��ѧ�Ծ��������棩 ���ͣ�̽����
ij�о���ѧϰС���ij����۽���������̽����
���������ϡ�������Ļ�ѧ�ɷ���Ҫ�ǣ�̼��Ƽ�����̼��þ�Ϳǽǵ���(�����ʵ�һ��)��
�ڿǽǵ��ײ�����ˮ������Ũ������Ⱥ����ɫ��
�ۼ��������ʵ���ǡ����Ƿۡ�����Ҫ�ɷ־���̼��ơ�
���������ۡ����������������ǽǵ������������������ ���� ���л����������������
�ڼ�������е�̼��ƺ���Ҫ��������۴�
��ʵ��̽�����о���ѧϰС���������������������
����һ ��������۵����
ȡ�����������Ʒ���Թ��У� �����۲쵽 ������������������ۡ�
������ �����������е�̼��ƺ���
������������������ͼ��ʾʵ��װ�á�ijѧ����סC�����ܣ���A��װ������ˮ��ȡ���ϲ����ӣ�����������A��ˮ����ȫ�����£�����Ϊ��װ���Ƿ�©���� ��
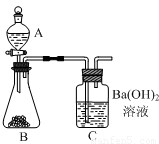
�����ȷ��ȡ6.000 g�������Ʒװ������B�У���A��װ��ϡ���ᡣ
�������B���������Ʒ�еμ�������ϡ���ᡣ������B��C�пɹ۲쵽������Ϊ �� ��
���������ȫ��Ӧ��C�е���Һ�� �� ������Ƶð�ɫ���������Ϊ11.820g�����������Ʒ��̼��Ƶ���������Ϊ100%��
��ʵ�鷴˼����ô˲ⶨ��������ܵ�ԭ���� ������������ţ�
����Ʒ�к���̼��þ
������δ�μ�����
��CO2�����ٶ�̫�쵼��δ��Ba(OH)2��ȫ����
��װ��B��ˮ������HCl�Ƚ���װ��C��
����չ���졿��ͬѧ����ڷ������в���Ҫװ��C��ֻҪ�õ�����ƽȷ����װ��A��B��Ӧǰ����������Ϳ��Եõ��������Ʒ��̼��Ƶĺ���������Ϊ�Ƿ���� ����˵������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����۳�Ϊ������ϲ����Ȼ��һЩ���������ñ��Ƿ�ð����������ۣ�һ�о���ѧϰС��Ϊ�˼���ij����۵���٣�����������̽����
����۳�Ϊ������ϲ����Ȼ��һЩ���������ñ��Ƿ�ð����������ۣ�һ�о���ѧϰС��Ϊ�˼���ij����۵���٣�����������̽�����鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com