���⻯ѧ��ȤС���ͬѧ����ij�������ķϼ�Һ����Ҫ�ɷ�ΪNa
2CO
3������������NaCl���������ʲ��ƣ���ʯ����Ϊԭ���Ʊ��ռ�������õ��ռ�ֲ�Ʒ�ijɷֽ��з����Ͳⶨ��
���ֲ�Ʒ�Ʊ���
��1�����ϼ�Һ��������Ũ�����γɽ�Ũ����Һ����ȴ����ʯ�����ϣ�������Ӧ�Ļ�ѧ����ʽΪ
Na2CO3+Ca��OH��2�T2NaOH+CaCO3��
Na2CO3+Ca��OH��2�T2NaOH+CaCO3��
��
��2������Ӧ��Ļ������ˣ��õ�����Һ���������ᾧ���Ƶ�NaOH�ֲ�Ʒ��
���ֲ�Ʒ�ɷַ�����
��1��ȡ�����ֲ�Ʒ����ˮ���μ�Ba��NO
3��
2��Һ���ְ�ɫ���ǣ�������Ӧ�Ļ�ѧ����ʽΪ
Ba��NO3��2+Na2CO3�TBaCO3��+2NaNO3
Ba��NO3��2+Na2CO3�TBaCO3��+2NaNO3
���ôֲ�Ʒ��һ��������
��������
��������
��������
�μ����ᱵ����������˵����Ʒ�к���̼���ƣ�̼���������������Ʒ�����Ӧ�����߲��ܹ���
�μ����ᱵ����������˵����Ʒ�к���̼���ƣ�̼���������������Ʒ�����Ӧ�����߲��ܹ���
��
��2����С��ͬѧͨ���Դֲ�Ʒ�ɷֵ�ʵ�������ȷ���ôֲ�Ʒ�к����������ʣ�
���ֲ�Ʒ��Na
2CO
3�����ⶨ��
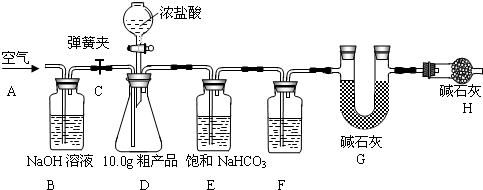
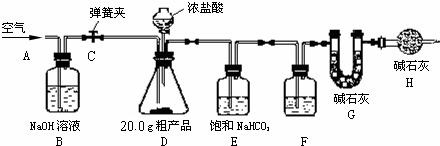
��1������ȤС���ͬѧ�������ͼ��ʾ��ʵ��װ�ã�ȡ10.0g�ֲ�Ʒ������ʵ�飮
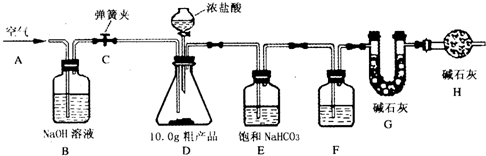
��2����������
�����Ӻ�װ�ã���������ԣ��ڴ��ɼ�C����A������ͨ��һ��ʱ��������۳���G���������ܹرյ��ɼ�C�������μ�Ũ������������ֱ��D��������ð�����ݴ��ɼ� C���ٴλ���ͨ��һ��ʱ����������ٴγ���G����������ǰ������������Ϊ0.48g��
��3������̽��
�ٲ���ڵ�Ŀ����
�ų�װ���ڵĿ�������ֹ���еĶ�����̼��ʵ������ɸ���
�ų�װ���ڵĿ�������ֹ���еĶ�����̼��ʵ������ɸ���
��
��Bװ�õ�������
���տ����еĶ�����̼
���տ����еĶ�����̼
��F�е��Լ�ӦΪ
Ũ����
Ũ����
��
��E װ�õ�������
���ճ�ȥ�ӷ������Ȼ�������
���ճ�ȥ�ӷ������Ȼ�������
����ط�Ӧ�Ļ�ѧ����ʽ��
NaHCO3+HCl�TNaCl+H2O+CO2��
NaHCO3+HCl�TNaCl+H2O+CO2��
��Hװ�õ�������
���տ����еĶ�����̼
���տ����еĶ�����̼
����û��Hװ�ã���ⶨ��Na
2CO
3������������
ƫ��
ƫ��
���ƫ����ƫС���������䡱������ʵ��10.0g�ֲ�Ʒֻ�ܲ���0.44g CO
2��������ϸ��������ʵ�飬����ʵ��ֵ0.48g����ȷֵ0.44gƫ���ԭ�����������ȷ��
װ��D��Ũ����ӷ������Ȼ�����װ��E��NaHCO3��Ӧ����������̼
װ��D��Ũ����ӷ������Ȼ�����װ��E��NaHCO3��Ӧ����������̼
��