
��7�֣���һ����ɫ�Ĺ����ĩ��������NaHCO3��NaOH�е�һ�ֻ���֣���ѧС�������ɽ�����ͼ��ʾ��ʵ�飺
���ϣ�1.�ƾ��Ƽ����¶�Լ500��600 �棻
2. NaOH 318.4���ۻ����ֽ⣬1390 ����ڲ��ֽ⣻
3. Na2CO3+CaCl2��CaCO3��+ 2NaCl
4. Na2CO3��Һ�ʼ��ԣ�CaCl2��Һ������
��1�����ʵ�鱨������ݣ�
| ʵ����� | ʵ������ | ʵ����� |
| ��ȡһ�����İ�ɫ��ĩ���ȣ�������������ͨ��С�ձ��У������������ʱ��ֹͣ���� ��С�ձ���ʢ�г����ʯ��ˮ�� | �Թ��ڱ�����ɫҺ�Σ�ʯ��ˮ����ǣ��Թ���ʣ���ɫ�������� | ��ĩ��һ������ ��Ӧ�Ļ�ѧ����ʽ�� |
| ��ȡʵ�����ʣ���ɫ�����������Թ��У����Թ��м���ϡ���� | | ʵ�����ʣ���ɫ������һ������ �� ��Ӧ�Ļ�ѧ����ʽ�� |
��1����NaHCO3 ��2NaHCO3��Na2CO3+ H2O+CO2 �����������
��Na2CO3��Na2CO3+2HCl��2NaCl+ H2O+CO2��
��2����ˮ�ܽ⣬���������CaCl2��Һʹ������ȫ�����ú����ϲ���Һ�еμӷ�̪��Һ������Һ��죬���������NaOH������Һ����ɫ���������û��NaOH��2�֣�
���������������1��
��2��Ϊȷ����ɫ��ĩ���Ƿ���NaOH������ȡ������ɫ��ĩ����ˮ�ܽ⣬���������CaCl2��Һʹ������ȫ�����ú����ϲ���Һ�еμӷ�̪��Һ������Һ��죬���������NaOH������Һ����ɫ���������û��NaOH��ʵ����� ʵ������ ʵ����� ��ȡһ�����İ�ɫ��ĩ���ȣ�������������ͨ��С�ձ��У������������ʱ��ֹͣ����
��С�ձ���ʢ�г����ʯ��ˮ���Թ��ڱ�����ɫҺ�Σ�ʯ��ˮ����ǣ��Թ���ʣ���ɫ�������� ��ĩ��һ������
NaHCO3 ��
��Ӧ�Ļ�ѧ����ʽ��2NaHCO3=Na2CO3+ H2O+CO2 ��ȡʵ�����ʣ���ɫ�����������Թ��У����Թ��м���ϡ���� ��������� ʵ�����ʣ���ɫ������һ������
Na2CO3��
��Ӧ�Ļ�ѧ����ʽ�ǣ�Na2CO3+2HCl=2NaCl+ H2O+CO2��
���㣺�����ƶϣ���ѧ����ʽ��̼�����ƺ�̼���Ƶ����ʡ�
�������ƶ����ʣ�Ҫ�������ʵ����ʣ���ƺ�����ʵ�飬ͨ�����еķ�Ӧ������ȷ��������������Ϣ�������Ŀ�еĿ��㣬�ǽ����Ĺؼ���


 �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
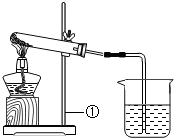 ��һ����ɫ�Ĺ����ĩ��������NaHCO3��NaOH�е�һ�ֻ���֣���ѧС�������ɽ�����ͼ��ʾ��ʵ�飺
��һ����ɫ�Ĺ����ĩ��������NaHCO3��NaOH�е�һ�ֻ���֣���ѧС�������ɽ�����ͼ��ʾ��ʵ�飺| ʵ����� | ʵ������ | ʵ����� |
| ��ȡһ�����İ�ɫ��ĩ���ȣ�������������ͨ��С�ձ��У������������ʱ��ֹͣ���� ��С�ձ���ʢ�г����ʯ��ˮ�� |
�Թ��ڱ�����ɫҺ�Σ�ʯ��ˮ����ǣ��Թ���ʣ���ɫ�������� | ��ĩ��һ������ ��Ӧ�Ļ�ѧ����ʽ�� |
| ��ȡʵ�����ʣ���ɫ�����������Թ��У����Թ��м���ϡ���� | ʵ�����ʣ���ɫ������һ������ ��Ӧ�Ļ�ѧ����ʽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�걱���л������п���ģ��ѧ�Ծ��������棩 ���ͣ�̽����
��7�֣���һ����ɫ�Ĺ����ĩ��������NaHCO3��NaOH�е�һ�ֻ���֣���ѧС�������ɽ�����ͼ��ʾ��ʵ�飺
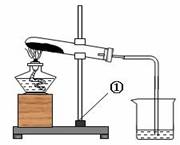
���ϣ�1.�ƾ��Ƽ����¶�Լ500��600 �棻
2. NaOH 318.4���ۻ����ֽ⣬1390 ����ڲ��ֽ⣻
3. Na2CO3+CaCl2��CaCO3��+ 2NaCl
4. Na2CO3��Һ�ʼ��ԣ�CaCl2��Һ������
��1�����ʵ�鱨������ݣ�
|
ʵ����� |
ʵ������ |
ʵ����� |
|
��ȡһ�����İ�ɫ��ĩ���ȣ�������������ͨ��С�ձ��У������������ʱ��ֹͣ���� ��С�ձ���ʢ�г����ʯ��ˮ�� |
�Թ��ڱ�����ɫҺ�Σ�ʯ��ˮ����ǣ��Թ���ʣ���ɫ��������
|
��ĩ��һ������
��Ӧ�Ļ�ѧ����ʽ��
|
|
��ȡʵ�����ʣ���ɫ�����������Թ��У����Թ��м���ϡ����
|
|
ʵ�����ʣ���ɫ������һ������ �� ��Ӧ�Ļ�ѧ����ʽ��
|
��2��Ϊȷ����ɫ��ĩ���Ƿ���NaOH�ķ����ǣ�ȡʵ�����ʣ���ɫ���������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
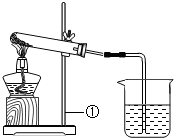 ��һ����ɫ�Ĺ����ĩ��������NaHCO3��NaOH�е�һ�ֻ���֣���ѧС�������ɽ�����ͼ��ʾ��ʵ�飺
��һ����ɫ�Ĺ����ĩ��������NaHCO3��NaOH�е�һ�ֻ���֣���ѧС�������ɽ�����ͼ��ʾ��ʵ�飺| ʵ����� | ʵ������ | ʵ����� |
| ��ȡһ�����İ�ɫ��ĩ���ȣ�������������ͨ��С�ձ��У������������ʱ��ֹͣ���� ��С�ձ���ʢ�г����ʯ��ˮ�� | �Թ��ڱ�����ɫҺ�Σ�ʯ��ˮ����ǣ��Թ���ʣ���ɫ�������� | ��ĩ��һ������ ______ ��Ӧ�Ļ�ѧ����ʽ�� ______ |
| ��ȡʵ�����ʣ���ɫ�����������Թ��У����Թ��м���ϡ���� | ______ | ʵ�����ʣ���ɫ������һ������ ______�� ��Ӧ�Ļ�ѧ����ʽ�� ______ |
�鿴�𰸺ͽ���>>
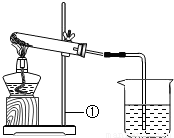
��Ŀ�����л�ѧ ��Դ��2011�걱���л������п���ѧ��ģ�Ծ��������棩 ���ͣ������
| ʵ����� | ʵ������ | ʵ����� |
| ��ȡһ�����İ�ɫ��ĩ���ȣ�������������ͨ��С�ձ��У������������ʱ��ֹͣ���� ��С�ձ���ʢ�г����ʯ��ˮ�� | �Թ��ڱ�����ɫҺ�Σ�ʯ��ˮ����ǣ��Թ���ʣ���ɫ�������� | ��ĩ��һ������ ______ ��Ӧ�Ļ�ѧ����ʽ�� ______ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com