
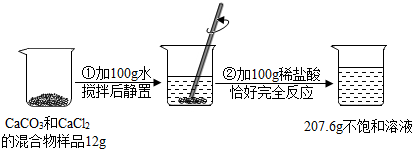
���� ��1�����������̼��Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���н��
��2������ͼ����Ϣ����֪������Ӧǰ��������ʵ�������Ϊ��12g+100g+100g=212g������Ӧ��ʣ�����ʵ�������Ϊ207.6g�����������غ㶨�ɿ���֪�����ʼ��ٵ�����Ϊ���ɶ�����̼���������������ɵĶ�����̼�������Ծݻ�ѧ����ʽ���̼��Ƶ��������н��
��3������̼��Ƶ������������̼��ƺ��Ȼ��Ƶ������Ƚ��н��
��4�����ݶ�����̼����������������Ȼ�������������������������������Լ���Ҫ36.5%��Ũ������������н��
��5��������������������ʽ�������ˮ���������н��
��� �⣺��1�������̼��Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼��������Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2�������CaCO3+2HCl�TCaCl2+H2O+CO2����
��2����Ӧ������CO2������Ϊ��12g+100g+100g-207.6g=4.4g��
�跴Ӧ��̼���������X���μӷ�Ӧ�Ȼ��������Ϊy�������Ȼ��Ƶ�����Ϊz��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73 111 44
X y z 4.4g
$\frac{100}{X}=\frac{44}{4.4g}$
X=10g
$\frac{73}{y}=\frac{44}{4.4g}$
y=7.3g
$\frac{111}{z}=\frac{44}{4.4g}$
z=11.1g
���$\frac{100}{X}=\frac{44}{4.4g}$��
��3�����ڻ������̼��Ƶ�������10g������ԭ�������Ʒ��CaCl2������Ϊ��12g-10g=2g������ԭ����������̼������Ȼ��������ȣ�10g��2g=5��1���ʶ��ߵ����������Ϊ5��1 �� 1��5�����5��1��1��5��
��4����Ҫ������������Ϊ36.5%��Ũ���������=$\frac{7.3g}{36.5%}$=20g�����20g��
��5��������ˮ������Ϊx��
$\frac{2g+11.1g}{207.6g-x}��100%$=10%
x=76.6g
����ڷ�Ӧ�����Һ������76.6gˮ�����ܵõ�������������Ϊ10%���Ȼ�����Һ��
���76.6��
���� Ҫ�����������Ŀ�����ȣ�Ҫ�������Ǹ��ݻ�ѧ��Ӧ����ʽ�ļ��㲽���ʽ���Լ���֮��ص�֪ʶ�ȣ�Ȼ���������������龰��ͼ����Ϣ�ȣ������ѧ�����֪ʶ�ͼ��ܣ�ϸ�µط������⣨��ͼ����Ϣ���ȸ�����Ϣ��Դ����ϸ�ĵ�̽��������������ĿҪ����������ѡ����ɣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ũ������ܶȴ���ˮ���ܶ� | |
| B�� | Ũ������ˮ���ʱ����Һ�¶�Ѹ������ | |
| C�� | Ũ������к�ǿ����ˮ�ԣ��������ڸ���CO��H2������ | |
| D�� | Ũ���ḯʴ�����Ƥ���������仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Һ�������9.5-10.5�� | B�� | ����֭��3.3-5.2�� | ||
| C�� | ����֭��2.0-3.0�� | D�� | ���ͣ�4.0-5.0�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ľ̿ | B�� |  ԭ�� | C�� |  ���ǡ����ǵ� | D�� |  �ɱ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 15.3% | B�� | 32.4% | C�� | 49.3% | D�� | 52.6% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
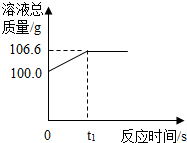 Ϊ�ⶨij������ʯ�����������������������ֺ�����ͬѧ��������һ����̼��10g������ʯ��Ʒ��ַ�Ӧ�����ʲ����뷴Ӧ�����������ɵ�������һ����������������Һ��ȫ���գ�����Һ�������뷴Ӧʱ��ı仯��ϵ��ͼ��
Ϊ�ⶨij������ʯ�����������������������ֺ�����ͬѧ��������һ����̼��10g������ʯ��Ʒ��ַ�Ӧ�����ʲ����뷴Ӧ�����������ɵ�������һ����������������Һ��ȫ���գ�����Һ�������뷴Ӧʱ��ı仯��ϵ��ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| Ԫ������ | �� | ̼ | �� | �� | �� | �� |
| Ԫ�ط��� | H | C | O | Cl | Na | Fe |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���� | �׳� | ��ѧʽ | ���ʷ��� | |
| A | �� | ˮ�� | Hg | ������ |
| B | �Ҵ� | �ƾ� | C2H5OH | �л��� |
| C | ̼���� | С�մ� | Na2CO3 | �� |
| D | ��̬������̼ | �ɱ� | CO2 | �л��� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com