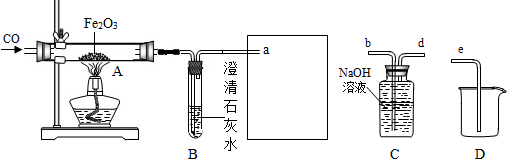
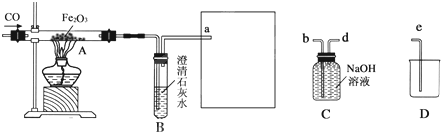
�⣺��1�����м�װ��װNaOHĿ���ǽ�һ����̼��������������̼���еĶ�����̼��ȥ������Ӱ��ʵ��������CO
2��NaOH ��Ӧ����Na
2CO
3��ˮ���ʻ�ѧ����ʽ��
CO
2+2NaOH=Na
2CO
3+H
2O
��2������װ���е����������Fe
XO
Y��ȫ������ԭ��ʣ����������Ϊ16.8g����֪���ɵ���������Ϊ16.8g���ֲ�ö�װ�õ�����������17.6g����֪���ɵ�CO
2����Ϊ17.6g����������ɵã�
YCO+Fe
XO
Y
XFe+YCO
2 56X 44Y
16.8g 17.6g
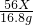
=
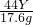
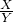
=
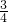
����Fe
XO
Y�Ļ�ѧʽΪ Fe
3O
4 ��3��û�м�װ�ã����лᵼ�¶�װ�õ�����ԶԶ���ӣ��Ӷ�ʹ����Ԫ�ص��������ӣ���ʹ�ⶨ�������Ԫ������Ԫ�ص������ı�ֵ ƫС
��4���������װ������װ�õ�˳������������ʵ���л���ˮ�����Ӷ�ʹ�����ǵ��������ӣ���ʵ������Ӱ�죮
��5���ɽ�����װ�ó�β���IJ��֣��ij��������ռ��������ռ�����δȼ��һ����̼�������ظ����ã�
����ȷ�𰸣���1��CO
2+2NaOH=Na
2CO
3+H
2O
��2��Fe
3O
4 ��3��ƫС
��4����
��5�����Խ�β�������õľƾ��ƻ�������
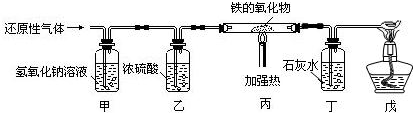
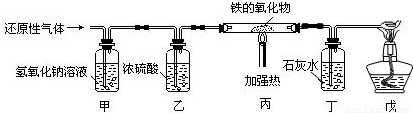
������������Ҫ����һ����̼�Ļ�ԭ����̽��һ�����������Fe
XO
Y������ɣ���ΪCO���л�ԭ�Կ��Խ������������е�����ԭ������Tͬʱ����CO
2����ΪCO���ж��ԣ�������ʵ����Ҫ��β��CO����������������ô���������װ�ý��з�������װ����Ƶ�Ŀ�ĺͲ��裬�Լ��ڴ�ʵ���е�ע�����Ȼ����н��
������������һ��̽����ʵ���⣬�����п����ѵ�Ҳ���ص㣻���Կα���һ����̼��ԭ������Ϊ���ݣ�������֪ʶ�ı仯�ǶȺ�Ǩ�������Ŀ��飬��ƽʱ��ѧϰ��Ҫע��ѧ���һ������ʵ��Ӧ�ã�CO�Ļ�ԭ�Կ��Խ������������е�����ԭ������ͬʱ���Ķ��ԣ�Ҫ�������������������Ⱦ��


 XFe+YCO2
XFe+YCO2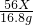 =
=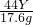
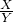 =
=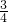


 ������������ϵ�д�
������������ϵ�д�