

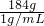 =184mL�����16��184��
=184mL�����16��184��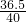 =
=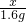 �����x=1.46g
�����x=1.46g ��100%=7.3%
��100%=7.3%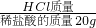 ��100%����Ҫ�����������������Ʒ�Ӧ�����Ȼ��ƺ�ˮ������ǡ����ȫ��Ӧʱ�������������Ƶ��������㷴Ӧ���������������
��100%����Ҫ�����������������Ʒ�Ӧ�����Ȼ��ƺ�ˮ������ǡ����ȫ��Ӧʱ�������������Ƶ��������㷴Ӧ���������������


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 12��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����
12��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����| ʵ����� | Ԥ������ | ���� |
ȡ��������������ϡ���� |
�����ݲ��� |
���������Na2CO3��������NaOH |
ȡ��������������ϡ���� |
û�����ݲ��� |
�����Dz���NaOH��������Na2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?��̨��ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨װ�����������ã����ȹر�ֹˮ�У�������������������Һ������ƿ�У�������յ�������̼���ٴ�ֹˮ�У�
��2012?��̨��ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨װ�����������ã����ȹر�ֹˮ�У�������������������Һ������ƿ�У�������յ�������̼���ٴ�ֹˮ�У�| ʵ����� | Ԥ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8%������������Һ��������ⶨijϡ���������ʵ�����������
ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8%������������Һ��������ⶨijϡ���������ʵ������������鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com