
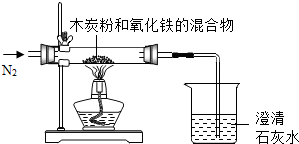
 ��֪ľ̿�ۺ�Fe2O3 ��Ӧ�Ļ�ѧ����ʽΪ��2Fe2O3+3C
��֪ľ̿�ۺ�Fe2O3 ��Ӧ�Ļ�ѧ����ʽΪ��2Fe2O3+3C
| ||
| ||
| ||
| 32 |
| 12+16��2 |
| 8N |
| 11 |
| ||
| Mg |
| 800N |
| 11M |
| 800N |
| 11M |


 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
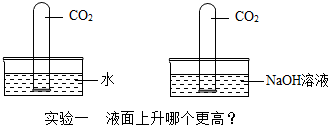 ���û�ѧ������գ�
���û�ѧ������գ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
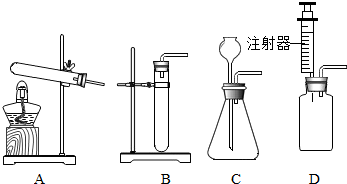
 ��1����ͼ��ͼA��ʵ���ҳ��õ����巢��װ��֮һ��д���ø�װ�ÿ���ȡ����������ķ�Ӧԭ�����û�ѧ����ʽ��ʾ��
��1����ͼ��ͼA��ʵ���ҳ��õ����巢��װ��֮һ��д���ø�װ�ÿ���ȡ����������ķ�Ӧԭ�����û�ѧ����ʽ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�� ���ʼ��� | B�� Ԫ�������彡�� |
| ��˿���Ϻͻ��˲��ϣ���ȼ������ζ �����Ǻ͵��ۣ���ơ��۲���ɫ�仯 |
������������٣�ʹ�����������ٻ��� ���������Ԫ�أ�Ԥ���������� |
| C����ȫ��ʶ | D����Դ�뻷�� |
| ���ʷ�������ȩ��Һ���� �����ڻ����������»�����ǰ������ |
����ˮ��Ⱦ������ʹ��ũҩ�뻯�� ���շϾɽ�������Լ��Դ�����ٻ�����Ⱦ |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com