�ִ��������Ʒ�ʵ�Ҫ��Խ��Խ�ߣ���ʳƷ����Ҫ��ɫ���㡢ζ����Ҫ��Ӫ����ʳƷ���Ӽ�Ӧ�˶��������õ�ʳƷ���Ӽ��У�
��1����ֹʳƷ���ܱ��ʶ����ӵķ��������籽���ᣨC6H5COOH���������г��õķ���������������________��Ԫ����ɣ�����һ�������к���________��ԭ�ӣ�
��2��Ϊ����ʳƷ�й٣���������ʳ�Ρ�С�մ����ǡ�ʳ�ȣ����г��������ɼ�����________����������ζ������________������ͬѧ����pH��ֽ���ʳ��pHֵ������pH��ֽ��ˮ��ʪ���ÿ��ӽ�ʳ����pH��ֽ�ϣ�����õ�pHֵ����ʵ�ʽ��________�������С������ȡ�����
��3������±�㶹����һ�オһ���������е���±��������Ϊ�ı�ʳƷ״̬�����ӵ����̼�����±����Ҫ�ɷ����Ȼ�þ����ˮ�к��зḻ��ʳ�κ��Ȼ�þ�������ᴿ������ͼ��ʾ��

����ͼʾ�ش𣺴����к��д�����ɳ���Ӵ�����ȡ���ε�ʵ�鲽����________��
д���١���������Ӧ�Ļ�ѧ����ʽ
��________����ܰ��ʾ�������ܽ��Ա�Ŷ������
��________��
��4�����У���ͼ��ʾ��ʳ���ж�������������Ӫ���������ʳ��BʳƷ������ЧԤ��________��________�ȼ����������������________������ţ�
�����ࣻ�ڵ����ʣ�����֬�Ļ�����λ������C���Ͽ��Բ���������谱���ᣮ
�⣺��1�����ݱ�����Ļ�ѧʽ C6H5COOH ��֪�������� C��H��O 3����Ԫ����ɣ�
���ݱ���Ԫ�ط������½ǵ����ֱ�ʾһ������������ԭ�ӵĸ������ɴ˿�֪������һ�������к��� 15��ԭ�ӣ�
�ʴ�Ϊ��3��15��
��2������С�մ��DZ��Ƹ��ʱ�ķ��ͷۣ����������ɼ�����С�մ�������ζ������ ���ǣ����ݲ�ij��Һ��PHֵʱ������ˮʪ��PH��ֽ���ٽ�������Һ�ε�PH��ֽ�ϣ��������Һ�������ԣ�pH��ƫ����˲�õ�pHֵ����ʵ�ʽ���� �ʴ�Ϊ��С�մ����ǣ���
��3�����ݴ�����ȡ���ε�ʵ�鲽���ǣ��ܽ⡢���ˡ��������ʴ�Ϊ���ܽ⡢���ˡ�������
�ٹ��˺���Һ�����Ȼ�þ��Һ����������������Һ������������þ��������ӦʽΪ��MgCl2+Ca��OH��2=Mg��OH��2��+CaCl2��
��������м������ᣬ�����Ȼ�þ����ӦʽΪ��Mg��OH��2+2HCl=MgCl2+2H2O��
�ʴ�Ϊ��MgCl2+Ca��OH��2=Mg��OH��2��+CaCl2�� Mg��OH��2+2HCl=MgCl2+2H2O��
��4����ͼ��֪BʳƷ�Ǹ߸Ƹ����̷ۣ����ƿ���Ԥ�����Ͳ����������ɵȣ���������Ԥ��ƶѪ�����������ɶ��ְ����ṹ�ɵļ�Ϊ���ӵĻ�����ʴ�Ϊ�����Ͳ����������ɵȣ���ƶѪ�� �ڣ�
��������1�����ݱ�����Ļ�ѧʽ C6H5COOH���з������
��2��С�մ��DZ��Ƹ��ʱ�ķ��ͷۣ��ǵ���ζ����ij��Һ��PHֵ�������з������
��3�������ᴿʵ�鲽���ǣ��ܽ⡢���ˡ����������ᴿ�Ĺ��̽��з������
��4������ʳƷ��Ӫ�����ý��з������
���������⿼��ѧ�����ݻ�ѧʽ�������ᴿʵ�鲽�裬��ij��Һ��PHֵ���з������⣬����֪ʶ���Ӧ�ã�
��ϰ��ϵ�д�
���ϰ��
��Ŀ�����л�ѧ
��Դ��
���ͣ���ѡ��
ijλͬѧ�Բ���ʵ�������¼���£�������ȷ����
- A.
�ྻ��ͭ˿�ھƾ��ƻ��������գ�ͭ˿������
- B.
þ���ڿ�����ȼ�գ�����ҫ�۵�ǿ�⣬��������þ
- C.
��ȼһ����̼����������ɫ���棬�ų���������
- D.
��ϡ����μӵ�pH��ֽ�ϣ���ֽ����ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ
��Դ��
���ͣ���ѡ��
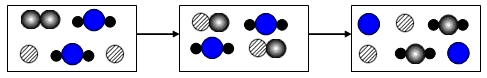
 ��ͼ��a��b���ֹ������ʵ��ܽ������ͼ������˵���в���ȷ����
��ͼ��a��b���ֹ������ʵ��ܽ������ͼ������˵���в���ȷ����
- A.
a���ܽ�ȴ���b���ܽ��
- B.
t1��ʱ��a��b���ܽ����ͬ
- C.
��a�л�������bʱ����������ȴ�ᾧ�ķ������ᴿa
- D.
��������ͬ��a��b������Һ��t2�潵��t1�棬b�����ľ����a��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ
��Դ��
���ͣ���ѡ��
�ο��������ʵ��۽ṹͼʾ�����������ӹ��ɵ�������
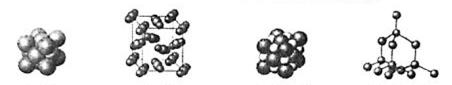
- A.
ͭ
- B.
�ɱ�
- C.
�Ȼ���
- D.
���ʯ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ
��Դ��
���ͣ���ѡ��
��֪ij���ӵĽṹʾ��ͼΪ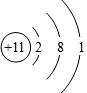 �������������
�������������
- A.
����Ԫ�ص�ԭ��
- B.
�ǽ���Ԫ�ص�ԭ��
- C.
������
- D.
������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ
��Դ��
���ͣ���ѡ��
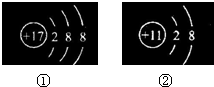 ������ͼ��ʾ�����ӽṹʾ��ͼ��˵���У�����ȷ����
������ͼ��ʾ�����ӽṹʾ��ͼ��˵���У�����ȷ����
- A.
�١��ڵĺ����������ͬ
- B.
��������Ԫ��
- C.
�١��ڶ�������ȶ��Ľṹ
- D.
����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ
��Դ��
���ͣ������
��������Դ��ȱ����ú̿���������������ú��Ϊ����������Ӱ�ȫ���������·���������˹��ը�������Ա�����������¹ʣ���ˣ�2005��10�¹��Ҿ�������������˽Ӫú�����ͣҵ���٣�����������ijѧУ��ѧ��ȤС������ӣ����Ƕ�ú����й�֪ʶ�����˲��ģ������������ѧ����֪ʶ�ش��������⣺
��1����˹����Ҫ�ɷ���________��������˹��ը��ԭ���û�ѧ����ʽ��ʾΪ��________��
��2���ڲ������ϵĹ����У���ȤС�鷢��úȼ�ղ����Ķ��������ǵ����������Ҫԭ��֮һ�����ǻ��˽������������KMnO4��Һ��Ӧ��ʹ��KMnO4��Һ��ɫ����Ӧ����ʽΪ��5SO2+2KMnO4+2H2O=2H2SO4+2MnSO4+X�������Ƴ�X�Ļ�ѧʽ________����Ӧ��
KMnO4��MnԪ�صĻ��ϼ�Ϊ________��
��3������ú��ʯ�͵Ȼ�ʯȼ�����ڲ���������Դ���������������ڻ���Ѱ������Դ������Ϊ�����Դ�㡢��Ⱦ�ٵ�����Դ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ
��Դ��
���ͣ������
ij�¶��£��Ȼ���������Һ����������Ϊ10%����ȡ162.5�˸��Ȼ���������Һ����82.2��һ��Ũ�ȵ���������ǡ�÷�Ӧ������
��1���Ȼ���������Һ�����ʵ�������
��2�����ɳ�����������
��3����Ӧ����Һ������������
�鿴�𰸺ͽ���>>





 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�




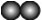

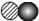
 ��ͼ��a��b���ֹ������ʵ��ܽ������ͼ������˵���в���ȷ����
��ͼ��a��b���ֹ������ʵ��ܽ������ͼ������˵���в���ȷ����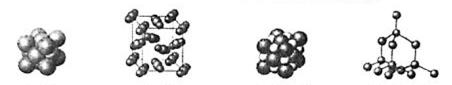
 �������������
�������������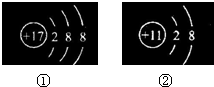 ������ͼ��ʾ�����ӽṹʾ��ͼ��˵���У�����ȷ����
������ͼ��ʾ�����ӽṹʾ��ͼ��˵���У�����ȷ����