�⣺����һ
��1����pH��ֽ�ⶨ��Һ��pHʱ����ȷ�IJ����ǣ��ò�����պȡ��Һ����PH��ֽ�ϣ��������ɫ�����վͿ���֪����Һ��PH��
��2����Ϊϡ������������Һʱ�����Լ�������Һ��PH��С��ֻ�����Ľӽ���7�����Dz��ܵ��ڻ�С��7�����PHС��7��˵���������ƺ����ᷢ���˻�ѧ��Ӧ��
������
��1��ʵ���С����ȡ��͡�����ֲ���͡�Ŀ���ǣ���ȥ��Һ���ܽ�����������ų������ĸ��ţ�
��2������1�����Լ��Ե�������ʹ��̪��Һ���ɫ��˵��̼������Һ�Լ��ԣ�
��3��������������Ϣ����֪������������������Һϡ�ͺ��ٵμӷ�̪�۲���������ʾ��ɫ��˵����ȡ����ҺŨ�ȹ���
������
��1���Ƚϵ�����͵�һ�������е�������������Ƶ�Ũ�ȿ���֪�������������������ƺ������Ũ�ȶ��ǵ�һ���2�������¶ȵı仯�ǵ�һ���4�����ڶ������һ��Ƚϣ�����������������䣬���������Ƶ�Ũ���ǵ�һ���2���������¶ȵĸı�Ҳ�ǵ�һ���2��������X=7
��2���������ƺ����ᷢ���кͷ�Ӧ����Һ���¶Ȼ����ߣ�������ƿ�ڵ�ѹǿ����ʹU�ιܵ�Һ����ָ߶Ȳ
��3������������������Ʒ�Ӧʱ������֪������μ��ٶȹ�����ܻ��������������
���ò����������ܹ���������������Ƴ�ַ�Ӧ��
��������������˵���������������Ʊ��ʣ�
�������������̼���ƣ����Կ��Եó�����ɷֿ�����̼���ƣ�̼���ƺ��������ƵȲ���
��4����1����2�������������������Ļ�ѧ��Ӧ������֪����̼���ƺ�����ķ�Ӧ��Ȼ����ݶ�����̼����������������̼���Ƶ�������Ȼ���ж��Ƿ����������ƽ����⣻
��3�������Ⱥ��������Ʒ�Ӧ��Ȼ��ź�̼���Ʒ�Ӧ���ɶ�����̼
�ʴ�Ϊ������һ����1����PH��ֽ���ڱ������ϣ��ò�����պȡ��������Һ������ֽ�ϣ�����ֽ��ɫ���ɫ�����ն���pH��
��2���ų���ϡ�Ͷ�ʹ��ҺpH��С�����أ�
����������1������������
��2���
��3����ȡ������Һ��ˮϡ�ͺ��ٵμ���ɫ��̪��
����Һ�ʺ�ɫ��
����������1��7����2��U����Һ������Ҹߣ�
��3���ٷ�ֹϡ�������
��ʹ��Ӧ���
������������Һ�к���̼����
��Na
2CO
3NaOH��Na
2CO
3�Ļ���
��4���⣺�������Ʒ��̼���Ƶ�����Ϊx����̼���Ʒ�Ӧ�����������Ϊy
Na
2CO
3+2HCl�T2NaCl+H
2O+CO
2��
106 73 44
x y��10% 2.2g
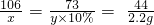
��ã�x=5.3g y=36.5g
NaOH��������13.3g-5.3g=8g
����������Ʒ�Ӧ�����������Ϊz
NaOH+HCl�TNaCl+H
2O��0.5�֣�
40 36.5
8g z��10%
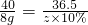
��� z=73g
�𣺣�1����Ʒ���������Ƶ�����Ϊ8g��
��2�����������Ʒ�Ӧ�����������Ϊ73g��
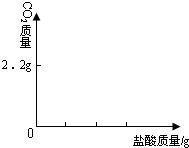
��3����ͼ�л������������ʾ������̼�������������ʾ����������Ĺ�ϵͼ����

����������һ����1����ȷ����pH��ֽ�ⶨ��Һ��pH��
��2���ڵμӹ��������û��Ӧ�����۽���������ϡ�͵�ʲô�̶ȶ�������С��7��
��������1��Ϊ����֤�Dz���������Ӱ�죬����Ҫ�ų������ĸ��ţ�
��2���������ƺͶ�����̼��Ӧ����̼���ƣ���̼����Ҳ�ܹ��Ƿ�̪��죬���ų������ֿ��ܣ�
����������1�����ݱ��е����ݽ��н��
��2�������кͷ�Ӧ�Ƿ��ȷ�Ӧ���з�����
��3������ʵ���еIJ���������ɵ�Ӱ��ͺ���������⣻
��4�������������������Ļ�ѧ��Ӧ������֪����̼���ƺ�����ķ�Ӧ��Ȼ����ݶ�����̼��������������̼���Ƶ�������Ȼ���ж��Ƿ����������ƽ��������⣮
������������Ҫ������ϡ���������������Һ�����кͷ�Ӧ�������ʵ�������ط���֤���ȷ�������ݣ����������кͷ�Ӧ�ĸ����Ӧ�ã�����pH��ֽ�ⶨ��Һ��pHʱ�IJ���������


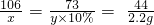
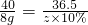



 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д�
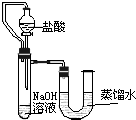
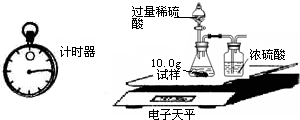


 ����֮�䷢����ѧ��Ӧʱ�������������Ե�������Щ��ѧ��Ӧȴ�۲첻�����Ե�����ij��ȤС��ͬѧΪ֤��NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ���Ӳ�ͬ�Ƕ����������ʵ�鷽����������ʵ�飮
����֮�䷢����ѧ��Ӧʱ�������������Ե�������Щ��ѧ��Ӧȴ�۲첻�����Ե�����ij��ȤС��ͬѧΪ֤��NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ���Ӳ�ͬ�Ƕ����������ʵ�鷽����������ʵ�飮