
 ��ѧ����ϵ�д�
��ѧ����ϵ�д� �ο�������ϵ�д�
�ο�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ����� | ʵ������ | ���� |
| ȡһ���� ���μӡ��������������� | �������� | ������ȷ |
| ȡ�����մ�ۼ�̼���Ʒ�ĩ���μӵμӡ��������������� | �� | ������ȷ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������ư�ɫ�Ļ��� |
| B�������Ϸ������һ�����ձ�ʱ�ܷ����ձ��ڱ�����ˮ�� |
| C���������洦�ۻ����Һ�� |
| D������ȼ��ʱŨ�̹��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| Һ������ | �״� | ����ˮ | ʳ����Һ | ����ˮ | ������Һ |
| �ϸ���֭Һ��ʾ����ɫ | ��ɫ | ����ɫ | ����ɫ | ��ɫ | ��ɫ |
| ��� | ������ | ���������� | ���������� |
| �ϸ���֭Һ��ʾ����ɫ | ��ɫ | ��ɫ | ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ��� | ���� | NaOH��Һ | ��t/�� |
| 1 | 3.65�� | 2.00�� | 3.5 |
| 2 | 3.65�� | 4.00�� | x |
| 3 | 7.30�� | 8.00�� | 14 |



| �� �� �� Ŀ | �� �� ʱ�� | ������g�� |
| ���� | | 10.00 |
| װ��+ϡ�������� | | 241.20 |
| װ��+ϡ��������+���� | ��Ӧ��ʼ��15�� | 249.20 |
| װ��+ϡ��������+���� | ��Ӧ��ʼ��35�� | 247.00 |
| װ��+ϡ��������+���� | ��Ӧ��ʼ��55�� | 247.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
 2Fe2O3 + 8SO2
2Fe2O3 + 8SO2�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
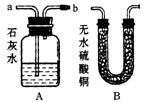
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com