
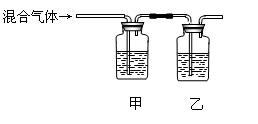
| | A | B | C | D |
| A | �� | �� | �� | �� |
| B | �� | �� | �� | �� |
| C | �� | �� | �� | �� |
| D | �� | �� | �� | �� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ���� | ���� | �Լ� | ���� |
| A | C02 | CO | 02 | ��ȼ |
| B | NaOH��Һ | Na2COs | �������� | �������� |
| C | CaCO3 | CaO | | ���� |
| D | Cu�� | Fe�� | ����ϡ���� | ���ˡ�ϴ�ӡ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ��������� | �����Լ��� | |
| ����1 | ����2 | ||
| A | ľ̿�ۺ�����ͭ | �۲���ɫ | ͨCO������ |
| B | NaCl��Һ��Na2CO3��Һ | ϡHCl | Zn |
| C | NH4HCO3���ʺ�K2SO4�ط� | ��Ca(OH)2��ĥ������ζ | ϡH2SO4 |
| D | ϡHCl��KCl��Һ | Fe2O3 | ��ɫ��̪ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ���� | ���� | ��ȥ���ʵķ��� |
| A | CaO | CaCO3 | ��������ϡ���������ٲ������� |
| B | KNO3 | NaCl | �Ƴ��ȱ�����Һ�����¡����ˡ�ϴ�ӡ����� |
| C | Cu�� | Fe�� | ��������ϡ���ᣬ���ˣ�ϴ�ӡ����� |
| D | CO | CO2 | �����建��ͨ��ʢ������������Һ��ϴ��ƿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
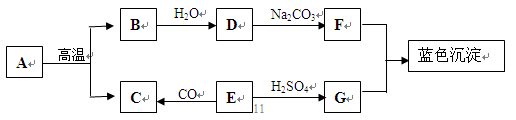
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
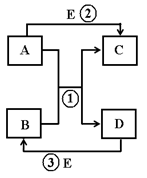
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ۢڢݢܢ� | B���ۢݢڢܢ� | C���ۢݢܢ٢� | D���ۢڢݢ٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
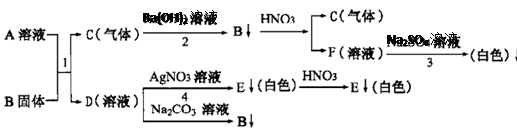
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com