
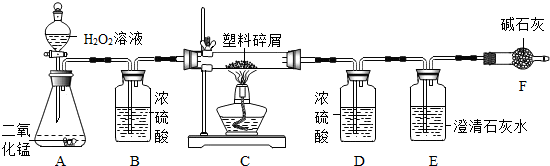
���� ��1��װ��B��Ҫ�����Ǹ������ã������ܳ��̴���
��2�����������ڶ������̴����³����¿�������������ˮ��
��3������������������̼����Ԫ����ɣ�����ȼ�պ����ɶ�����̼��ˮ��
��4��������Ԫ�������غ���н�𣮷���ʽ��д�ؼ�Ҫע����������ƽ��
��5����ĺ�����ͨ�����ɵ�ˮ������������ģ�
��� �⣺��1��װ��B�Ǽ���ƿ�������ܳ�����������ϴ�������ã�ͬʱ���岻�ܽ������װ�ã����Ը����ǣ�B�еĵ���Ӧ�����̳���
��2�����������ڶ������̴����³����¿�������������ˮ����Ӧ�ķ���ʽ�ǣ�2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��3����������̼����Ԫ����ɣ�����װ��Cȼ�պ����ɶ�����̼��ˮ��������̼ͨ��ʯ��ˮ���ɳ�����ʹ��Һ����ǣ���Ӧ�ķ���ʽ�ǣ�CO2+Ca��OH��2�TCaCO3��+H2O������ʵ��������ǣ�����ʯ��ˮ����ǣ�
��4��������Ԫ�������غ�֪����������Ԫ�ص�������Ϊˮ����Ԫ�ص�������7.2��$\frac{2}{18}$=0.8g��
��5����Ԫ�ص�������ͨ��װ��D����ˮ���ⶨ�ģ����û��Bװ�ã���ʹװ��D���������������غ㶨��֪����Ԫ������Ҳ������
�ʴ�Ϊ����1��B�еĵ���Ӧ�����̳�����2��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2������3������ʯ��ˮ����ǣ�CO2+Ca��OH��2�TCaCO3��+H2O��4��0.8����5��ƫ��
���� ������ʵ��̽���⣬�����˻�������̼Ԫ�غ���Ԫ�ص���֤���̺��ݻ�ѧʽ�ļ��㣬�ۺ��Լ�ǿ��ֻҪ���巴Ӧ��ʵ�ʽ������⣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ˮʵ���п�ֱ�ӹ۲쵽�������ǣ�ˮ���⡢������Ԫ����� | |
| B�� | ����������Ƶ������к������������������������ | |
| C�� | ������������ͭ��������ϡ���ᷴӦ�Ľ��������� | |
| D�� | ��ʯ�ҵ���Ҫ�ɷ��������ƣ���ʯ������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ���ʣ����ʣ� | �������� |
| A | CO2��H2O�� | ������ͨ��ʢ�л��ĸ���� |
| B | Cu��CuO�� | ͨ���������������� |
| C | CO2��CO�� | ��ȼ |
| D | FeCl2��CuCl2�� | ����������м����ַ�Ӧ����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ȼ�ŵľƾ��Ʋ���������������ʪ������ | |
| B�� | ������������ʱ��û�������κ����������������Ѵ��� | |
| C�� | ����100g 10%���Ȼ�����Һ��Ҫ10g �Ȼ��ƺ�100g ˮ | |
| D�� | ��pH��ֽ�ⶨ��Һ����ʱ���Ƚ�pH��ֽ��ˮ��ʪ��Ȼ���ٲⶨ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������ȼ�� | B�� | ����������ȷֽ� | ||
| C�� | ʯ���ڿ�����ȼ�� | D�� | ��˿��������ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
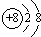 ��C+�ĺ�������Ų�����ԭ����ͬ��DԪ�ص�ԭ�����������Ӳ㣬��һ��͵�����������ĺͱȵڶ����������1���Իش�
��C+�ĺ�������Ų�����ԭ����ͬ��DԪ�ص�ԭ�����������Ӳ㣬��һ��͵�����������ĺͱȵڶ����������1���Իش��鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com