
 ×100%�����ܼ���������������������ʱ����Һ������������������
×100%�����ܼ���������������������ʱ����Һ������������������ ×100%������60��ʱ������Һ����������������
×100%������60��ʱ������Һ����������������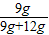 ×100%��42.9%��75%����C����ȷ��
×100%��42.9%��75%����C����ȷ�� ×100%�����ݸù�ʽ����ͨ��������Һ��ɵı仯���ж϶���Һ����������������Ӱ�죮
×100%�����ݸù�ʽ����ͨ��������Һ��ɵı仯���ж϶���Һ����������������Ӱ�죮 

 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
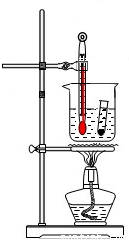
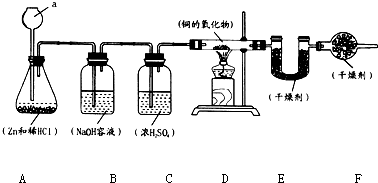 ʵ������п��ϡ������ȡ����������������ԭͭ��һ���������ʵ�����ⶨ�����������ɣ�ʵ��װ����ͼ��ʾ��
ʵ������п��ϡ������ȡ����������������ԭͭ��һ���������ʵ�����ⶨ�����������ɣ�ʵ��װ����ͼ��ʾ��7.2g-0.9g��
| ||
0.9g��
|
7.2g-0.9g��
| ||
0.9g��
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?�γ�ģ�⣩ʵ���ҳ��ñ��������������Ȼ����Һ��Ӧ��ȡ�����ĵ�������Ӧ�Ļ�ѧ����ʽΪ��NaNO2+NH4Cl=NaCl+N2+2H2O���˷�Ӧ�Ƿ��ȷ�Ӧ����ʵ��װ����ͼ��ʾ���Իش�
��2013?�γ�ģ�⣩ʵ���ҳ��ñ��������������Ȼ����Һ��Ӧ��ȡ�����ĵ�������Ӧ�Ļ�ѧ����ʽΪ��NaNO2+NH4Cl=NaCl+N2+2H2O���˷�Ӧ�Ƿ��ȷ�Ӧ����ʵ��װ����ͼ��ʾ���Իش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijͬѧ��Ƶ���һ����̼��ԭ��������ʵ��װ����ͼ��ʾ���Իش��������⣺
ijͬѧ��Ƶ���һ����̼��ԭ��������ʵ��װ����ͼ��ʾ���Իش��������⣺
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2006?������ijͬѧ��Ƶ���һ����̼��ԭ��������ʵ��װ����ͼ��ʾ��
��2006?������ijͬѧ��Ƶ���һ����̼��ԭ��������ʵ��װ����ͼ��ʾ��
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijͬѧ��Ƶ���һ����̼��ԭ��������ʵ��װ����ͼ��ʾ���Իش��������⣺
ijͬѧ��Ƶ���һ����̼��ԭ��������ʵ��װ����ͼ��ʾ���Իش��������⣺�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com