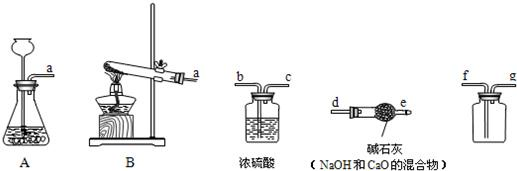
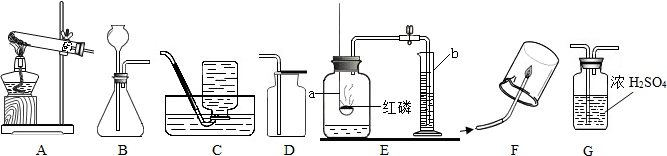
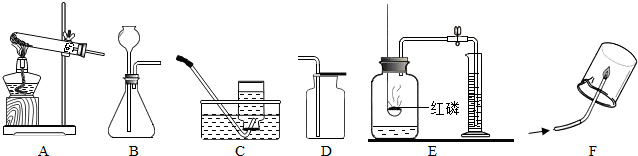
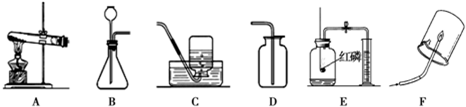
�⣺��1��ʵ�����ø�����ؼ��ȷֽ���������غͶ������̺���������Ӧ�ķ���ʽΪ��2KMnO
4
K
2MnO
4+MnO
2+O
2�����÷�Ӧ�ķ���װ�����ڹ̹̼����ͣ���ѡ��A��
��2��ʵ�����Ʊ�������̼��ʯ��ʯ��ϡ��������Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�ķ���ʽ�ǣ�CaCO
3+2HCl=CaCl
2+H
2O+CO
2������Ϊ������̼������ˮ���ܶȱȿ����ʲ��������ſ������ռ���
��3����������ʱӦ�������ǵ�ľ������ƿ�ڣ���ľ����ȼ��˵�����ռ�����
��4���ú��ײⶨ�����е����������ú��������ܱ������ڵ�����ʹװ���ڵ���ѹ��С�����������µ��������ⶨ�ģ��ʴ�Ϊ��������
��5�����ݻ�ѧ��Ӧǰ��Ԫ�ص�������֪������ȼ��ʱ���е�̼Ԫ��ת���ɶ�����̼����Ԫ��ת����ˮ�����Իῴ���������dz���ʯ��ˮ����ǣ������ձ��ڱ�����ɫҺ�Σ���˵���ж�����̼��ˮ�����ɣ��Ӷ�Ҳ˵���˼�������̼Ԫ�غ���Ԫ����ɵģ�
�ʴ�Ϊ����1��2KMnO
4
K
2MnO
4+MnO
2+O
2�� A
��2��CaCO
3+2HCl=CaCl
2+H
2O+CO
2�� D
��3��ľ����ȼ ��4������
��5������ʯ��ˮ����ǣ������ձ��ڱ�����ɫҺ��
��������1��ʵ�����ø�����ؼ��ȷֽ���������غͶ������̺��������÷�Ӧ�ķ���װ�ø��ݷ�Ӧ���״̬�ͷ�Ӧ����ѡ��
��2��ʵ�����Ʊ�������̼��ʯ��ʯ��ϡ��������Ȼ��ơ�ˮ�Ͷ�����̼�����ݶ�����̼������ˮ���ܶȱȿ�����ѡ���ռ�װ�ã�
��3����������ʱӦ�������ǵ�ľ������ƿ�ڣ���ľ���Ƿ�ȼ��
��4�������ú��ײⶨ�����е�����ԭ��������
��5�����������غ㶨���л�ѧ��Ӧǰ��Ԫ�ص�����������
������������Ҫ������������ȡ�ķ�Ӧԭ��������Ԫ���غ㷨֤�����ʵ���ɣ������ص㣬���ǿ����ȵ㣬������Ŀ������ʽ������Ҫ��ѧ����������Ļ������������ã�

 K2MnO4+MnO2+O2�����÷�Ӧ�ķ���װ�����ڹ̹̼����ͣ���ѡ��A��
K2MnO4+MnO2+O2�����÷�Ӧ�ķ���װ�����ڹ̹̼����ͣ���ѡ��A�� K2MnO4+MnO2+O2�� A
K2MnO4+MnO2+O2�� A

 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�