
���� ��1��������Ʒ�еĺ�ͭ��ֱ�Ӽ���������ͭ��������
��2����������ͭ��������ϻ�ѧ����ʽ�������ɵ�ͭ��������������Ʒ������ͭ��������
��3����������ͭ�������Ͷ�Ӧ�Ļ�ѧ����ʽ�������ĵ�ϡ���������ʵ������Լ�����ͭ�����������������������㣮
��� �⣺ԭ40t������ͭ��Ʒ��������ͭ������=40t����1-10%��=36t
�����ĵ������е����ʵ�����Ϊx�����ɵ�����ͭ������Ϊy�����ɵ�ͭ������Ϊz
Cu2O+H2SO4�TCuSO4+Cu+H2O
144 98 160 64
36t x y z
$\frac{144}{36t}$=$\frac{98}{x}$=$\frac{160}{y}$=$\frac{64}{z}$
x=24.5t
y=40t
z=16t
��ͭ������Ϊ40t��10%+16t=20t
��������ʵ���������Ϊ20%��ϡ���������Ϊ24.5t��20%=122.5t
��Ӧ��ʣ�����Һ������Ϊ122.5t+36t-16t=142.5t
10%������ͭ��Һ������Ϊ40t��10%=400t
��Ҫ�����ˮ������Ϊ400t-142.5t=257.5t
�𣺣�1��ԭ40t������ͭ��Ʒ��������ͭ������Ϊ36t��
��2����Ӧ�����ϴ�Ӻ�ɿɵø�����ͭ������Ϊ20t��
��3���������ʵ���������Ϊ20%��ϡ��������Ϊ122.5t����ˮ������257.5t��
���� ���ݻ�ѧ����ʽ����ʱ����һҪ��ȷ��д��ѧ����ʽ���ڶ�Ҫʹ����ȷ�����ݣ������������Ҫ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
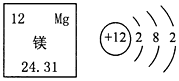
| A�� | þ�����ԭ��������24.31 | B�� | þԭ���ڻ�ѧ�仯�����õ����� | ||
| C�� | þԪ�����ڽ���Ԫ�� | D�� | þԭ�ӵĺ˵����Ϊ12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | N2O | B�� | NO2 | C�� | N2O5 | D�� | N2O3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ͥ��ˮ���м��д����ŷ� | B�� | ��ҵ��ˮ������ѭ������ | ||
| C�� | ũ������ô�ˮ���� | D�� | �������ռ���С����ˮ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | �ٺ͢��������ӵĻ�ѧ�������� | B�� | �ں͢۱�ʾ�����Ӿ�Ϊ���� | ||
| C�� | �ۺ͢�����ͬ��Ԫ�صIJ�ͬ���� | D�� | �۱�ʾ�����ӵķ���ΪMg+2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| 26-A | 26-B |
| ��1����ԭ��ת��Ϊ�����ӣ� ������������С������С������ ��2�������£��Ȼ��Ʊ�����Һ�У��Ȼ�����ˮ��������Ϊ9��25 �� ����֪�������£��Ȼ��Ƶ��ܽ��Ϊ36g�� | ��1�������Ȼ��Ƶ�����Na+��Cl-���������ţ��� ��2������ʹ�ù����У��Ȼ�����Һ�����ʵ����������ı䣨��ı䡱���䡱�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com