
(�˲���2006)һ��ѧС��Ϊ�˲ⶨijͭ���м�ʽ̼��ͭ[ ]��������������ȡ��ͭ����Ʒ30g���ձ��У���ε�����������Ϊ10����ϡ�������պ���ȫ��Ӧ������ȥϡ����146g(����ͭ���е����ʲ���ϡ���ᷴӦ��Ҳ������ˮ)����
]��������������ȡ��ͭ����Ʒ30g���ձ��У���ε�����������Ϊ10����ϡ�������պ���ȫ��Ӧ������ȥϡ����146g(����ͭ���е����ʲ���ϡ���ᷴӦ��Ҳ������ˮ)����
(1)ϡ������HCl�������Ƕ��ٿ�?
(2)ͭ���м�ʽ̼��ͭ�������Ƕ��ٿ�?�����������Ƕ���?
(3)��Ӧ�������Ȼ�ͭ��Һ�����ʵ����������Ƕ���?
[��Ӧ�Ļ�ѧ����ʽΪ��
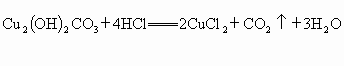
��Ӧ�и����ʵ���Է���������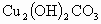 -222
-222
HCl-36.5����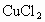 -135����
-135���� -44����
-44����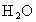 -18
-18

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�013
(
�˲���2006)С������ͼ��ʽ����ѧ֪ʶ���й��ɣ����мװ������ҡ��������������й�ϵ�У��д����һ����[
����]

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2006�����ʡ�˲����п���ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�걱�����п���ѧһģ����Ԥ���Ծ���һ���������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com