
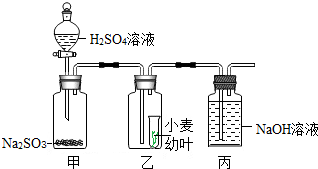
 ����г���һЩ�����̷�ʹ�õ��ж���SO2�����ijЩʳƷ����Ư�ף�ij�Ƽ���ȤС����С����ҶΪʵ������о�SO2��ֲ���Σ��������ͨ���������ϣ���֪������Һ���̬���������Ʒ�Ӧ�ɲ���SO2���壺Na2SO3+H2SO4�TNa2SO4+SO2��+H2O���ʵ����ͼ��ʾ��
����г���һЩ�����̷�ʹ�õ��ж���SO2�����ijЩʳƷ����Ư�ף�ij�Ƽ���ȤС����С����ҶΪʵ������о�SO2��ֲ���Σ��������ͨ���������ϣ���֪������Һ���̬���������Ʒ�Ӧ�ɲ���SO2���壺Na2SO3+H2SO4�TNa2SO4+SO2��+H2O���ʵ����ͼ��ʾ������ ��1������Һ�����������������������������������ļ��㹫ʽ���Լ��������������
��2�������ɶ���������������ݻ�ѧ����ʽ���Լ�����μӷ�Ӧ������������Һ��������
��� �⣺��1�������������Ϊ200g��75%=150g�����150��
��2������Ҫ����������Һ������Ϊx��
Na2SO3+H2SO4=Na2SO4+SO2��+H2O
126 64
x��10% 0.64g
$\frac{126}{x��10%}=\frac{64}{0.64g}$
���x=12.6g
����Ҫ����������Һ������Ϊ12.6g��
���� ���⿼���������������ļ��㡢�йػ�ѧ����ʽ�ļ��㡢��Ļ�ѧ���ʡ�ʵ��̽�����������Σ����֪ʶ���Ѷ��Դ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ˮ����������ͻ�ѧʵ��������ʮ����Ҫ�����ã�
ˮ����������ͻ�ѧʵ��������ʮ����Ҫ�����ã��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
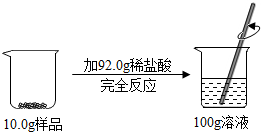 ij��Ʒ������ͭ��ͭ��ɣ�ȡ10.0g����Ʒ���ձ��У������м���92.0gϡ���ᣬǡ����ȫ��Ӧ������������Һ����Ϊ100.0g������ѧ��Ӧ����ʽΪ��CuO+2HCl�TCuCl2+H2O��
ij��Ʒ������ͭ��ͭ��ɣ�ȡ10.0g����Ʒ���ձ��У������м���92.0gϡ���ᣬǡ����ȫ��Ӧ������������Һ����Ϊ100.0g������ѧ��Ӧ����ʽΪ��CuO+2HCl�TCuCl2+H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | С��45% | B�� | ����45% | C�� | ����45% | D�� | ��ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ˮ�ӷ� | B�� | ú������ | C�� | ��̥��ը | D�� | �ɱ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| ѡ�� | ���ʣ�������Ϊ���ʣ� | �Լ� | �������� |
| A | CaO ��CaCO3�� | -- | ���ȵ��������ټ��� |
| B | CO2���壨HCl�� | ����������Һ��Ũ���� | ϴ�������� |
| C | NaCl���壨BaSO4�� | ˮ | �ܽ⡢���ˡ����� |
| D | NaCl���壨MgCl2�� | NaOH��Һ | ���ˡ����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� |  ��ƯҺ |  ����� |  �ܵ�ͨ |
| �� �� | Ưϴ���ʹɫ������ | ����۹�������ζ | �ٵ���ͨ�������� |
| ��Ч�ɷ� | �������� | ���ᣨHCl�� | �������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com