�⣺��1�����ݷ�Ӧ�Ļ�ѧ����ʽ5CH
3OH+12O
2+6NH
3
3B+5CO
2+19H
2O����Ӧ����̼���⡢������ԭ�Ӹ����ֱ�Ϊ5��38��29��6����Ӧ�����������̼���⡢������ԭ�Ӹ����ֱ�Ϊ5��38��29��0�����ݷ�Ӧǰ��ԭ�����ࡢ��Ŀ���䣬��3B�к���6����ԭ�ӣ���ÿ��B������2����ԭ�ӹ��ɣ�������X�Ļ�ѧʽΪN
2��
��2�����ݼ״��Ļ�ѧʽCH
3OH��֪��̼Ԫ�ء���Ԫ�غ���Ԫ�ص�������=��12��1������1��4������16��1��=3��1��4��
��3�����ݼ״��Ļ�ѧʽCH
3OH��֪���״�����Ԫ�ص���������=
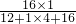
��100%=50%��
��4�������������=��������ijԪ�ص������¸�Ԫ�ص���������������״�������Ϊ36g��
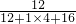
=96g��
�ʴ�Ϊ����1��N
2����2��3��1��4����3��50%����4��96g��
��������1���������غ㶨�ɣ���Ӧǰ��ԭ�����ࡢ��Ŀ�����䣬�ݴ��ɷ�Ӧ�Ļ�ѧ����ʽ�ƶ�������B�Ļ�ѧʽ��
��2�����ݻ������и�Ԫ��������=��ԭ�ӵ����ԭ��������ԭ�Ӹ���֮�ȣ����з������
��3�����ݻ�������Ԫ�ص���������=
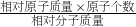
��100%����������
��4�����ݻ�������ijԪ�ص�����=�û��������������Ԫ�ص����������������������=��������ijԪ�ص������¸�Ԫ�ص��������������з������
�����������ѶȲ�����ͬѧ�ǽ������Ϣ��������������غ㶨�ɡ���ѧʽ���йؼ�����з������⡢��������������

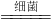 3B+5CO2+19H2O
3B+5CO2+19H2O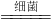 3B+5CO2+19H2O����Ӧ����̼���⡢������ԭ�Ӹ����ֱ�Ϊ5��38��29��6����Ӧ�����������̼���⡢������ԭ�Ӹ����ֱ�Ϊ5��38��29��0�����ݷ�Ӧǰ��ԭ�����ࡢ��Ŀ���䣬��3B�к���6����ԭ�ӣ���ÿ��B������2����ԭ�ӹ��ɣ�������X�Ļ�ѧʽΪN2��
3B+5CO2+19H2O����Ӧ����̼���⡢������ԭ�Ӹ����ֱ�Ϊ5��38��29��6����Ӧ�����������̼���⡢������ԭ�Ӹ����ֱ�Ϊ5��38��29��0�����ݷ�Ӧǰ��ԭ�����ࡢ��Ŀ���䣬��3B�к���6����ԭ�ӣ���ÿ��B������2����ԭ�ӹ��ɣ�������X�Ļ�ѧʽΪN2��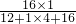 ��100%=50%��
��100%=50%��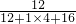 =96g��
=96g��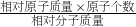 ��100%����������
��100%����������



 ��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺
��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺