
���� �������֪������ƷӦΪ�������ƺ�̼��ƵĻ��������������Ƶ�ˮ��Һ�ʼ��ԣ��������÷�̪��Һ�����𣻶�̼�������Ժ�����Һ��Ӧ�����ݲ�����������ϡ���������������ⲻ�ѿ�������Ʒ�����������ƴ��ڣ���Ӧ����Ʒ��ˮ��Һ�еμӷ�̪��Һ����̪��Һ���죻��Ʒ����̼��ƴ��ڣ���Ӧ����Ʒ�еμ�ϡ���ᣬ������ð����
��� �⣺֤���������ƵĴ���һ������Һ�н��з�Ӧ�������Ȱ���Ʒ�ܽ⣻֤��̼��ƵĴ���һ���ѡ������Һ���ʿ�ѡ�õ��Լ���ϡ���ᣮ�������֪��Ʒ�����������ƺ�̼��ƣ����������Ƶ�ˮ��Һ��ʹ��̪��Һ��죬��̼����ܺ�ϡ���ᷴӦ���ɶ�����̼���壮
�ʴ�Ϊ����1����һ�ྻ���Թ��м�����������Ʒ����ˮ�����裬�μ�����ɫ��̪��Һ����Һ��죬˵����Ʒ�����������ƴ��ڣ���2����ȡ������Ʒ��һ�ྻ�Թ��У��μ�������ϡ���ᣬ�д�������ð����˵����Ʒ����̼��ƴ��ڣ�
���� ������Ҫ�������������ƺ�̼��Ƶ�ijЩ��ѧ���ʣ��˽��̪��Һ�����ʣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
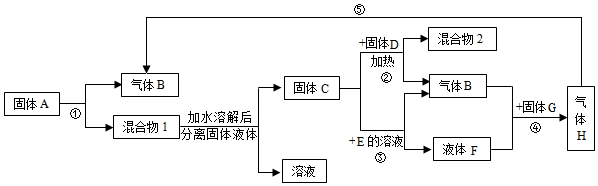
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ѧ����������ϢϢ��أ�
��ѧ����������ϢϢ��أ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������������ĺ���ʱ����ȼ����ҪѸ�����뼯��ƿ�� | |
| B�� | ��˫��ˮ�ӷ�Һ©��Ѹ�ټ���װ�������������̷�ĩ����ƿ�� | |
| C�� | �ѵ�ȼ��ľ̿����ȼ�ճ��ڣ����϶��»������뼯�������ļ���ƿ�� | |
| D�� | ���Թ��м��뼸С�����ʯ����ƽ�����Թܿڣ��ٻ�����ֱ�Թ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | ���� | C�� | ˮ | D�� | ��̪ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
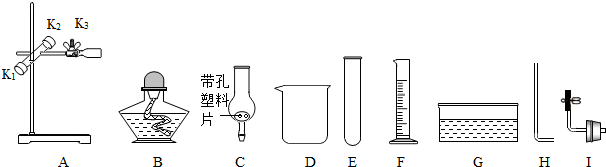
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com