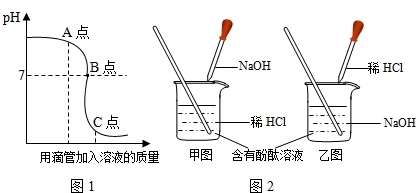
�кͷ�Ӧ��ҽҩ������ũҵ�ϲ������Ź㷺��Ӧ�ã�ͼ1��ʾ�����NaOH��Һ������Ӧ��������Һ��pH�������Һ��������ϵ���������ͼ1�л�ȡ��Ϣ���ش��������⣺
��1��pH��ֽ��ʹ�÷�����
˺һС��pH��ֽ���ڱ������У��ò�����պȡ��Һ����pH��ֽ�ϣ��������ɫ�����ն���pHֵ
˺һС��pH��ֽ���ڱ������У��ò�����պȡ��Һ����pH��ֽ�ϣ��������ɫ�����ն���pHֵ
��
��2��ʵ������ǰ�������
��
��
�����ң�ͼ��ʾ���У�
��3��ͼ��A��ʱ����Һ�����ʵĻ�ѧʽΪ
NaOH��NaCl
NaOH��NaCl
��
��4����֪100g ������������Ϊ7.3%��ϡ��������100gNaOH��Һǡ����ȫ��Ӧ����
�ٸ�NaOH��Һ����������������
8%
8%
��
�ڵ�pH=7ʱ���ձ���δ�������������������Һ���������������Ƕ��٣�����д���淶�Ľ��ⲽ�裩




 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�

