



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�걱���в�ƽ�����꼶��ѧ����ĩ������⻯ѧ�Ծ��������棩 ���ͣ�̽����
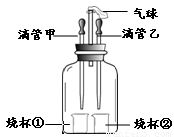
��ͼװ�ÿ�����ɶ����ʵ�飬����ҩƷ�����١�ʵ���������ԡ�β�������ݵ��ŵ㡣
С���ϣ�Na2CO3 + H2SO4=Na2SO4 + H2O + CO2����
�����ƣ��׳���ʯ�ң���ˮ��Ӧ���ȡ�
��1������������Ũ��ˮ������������ɫ��̪��Һ�����ס����е�Һ��ͬʱ������һ��ʱ��۲쵽��ɫ��̪��Һ��죬˵�����Ӿ��е�������________��
��2������������ˮ����������ϡ���ᣬ�ձ�����ʢ����ʯ����ҺȾ����ɫ�ĸ���ֽ�����ձ�����ʢ��̼���Ʒ�ĩ��Ϊ����֤ʹʯ���ɫ��������̼������Ƕ�����̼��Ӧ��ȡ��ʵ�������________��
��3�����Ƚ����е�Һ�強�����۲쵽�������Թ���һ��ʱ���ָ�ԭ״���ٽ����е�Һ�強�������������Թ����뽫�±��е��Լ�����������
|
|
�ιܼ� |
�ձ��� |
��� |
�ձ��� |
|
�Լ� |
H2O |
|
|
MnO2 |
�����������Ϊװ������ѹ����������ѹ�����ԭ��������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ�����з�خ��ʯ����ѧ���꼶���£��¿���ѧ�Ծ���8��9��10��Ԫ���������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com