
����Լռ����������71%������ʮ�־�
�Ŀ���DZ������±�Ǻ�ˮ��ȡʳ�κ�IJ�Һ������
��±�����Ƶý���þ�Ȼ���ԭ�ϡ���ͼ������ij��
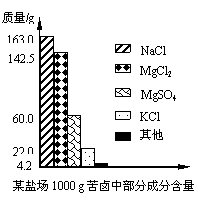
����±�в��ֳɷֺ�������ͼ���Լ��㣺
(1) ��ʹ100 g�ÿ�±�е�MgCl2��MgSO4��ȫת��ΪMg(OH)2��������Ҫ20% NaOH��Һ���ٿˣ�
 (2)������������ȫת��Ϊ��ˮMgCl2����������״̬�½��е�⣬�ɵý���þ���ٿˣ��������������������������ܶ�Ϊ3.0 g/L����
(2)������������ȫת��Ϊ��ˮMgCl2����������״̬�½��е�⣬�ɵý���þ���ٿˣ��������������������������ܶ�Ϊ3.0 g/L����
 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
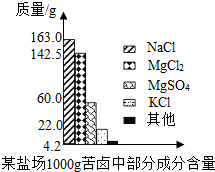 ����Լռ����������71%������ʮ�־�Ŀ���DZ������±�Ǻ�ˮ��ȡʳ�κ�IJ�Һ�����ÿ�±�����Ƶý���þ�Ȼ���ԭ�ϣ���ͼ������ij�γ���±�в��ֳɷֺ�������ͼ���Լ��㣺
����Լռ����������71%������ʮ�־�Ŀ���DZ������±�Ǻ�ˮ��ȡʳ�κ�IJ�Һ�����ÿ�±�����Ƶý���þ�Ȼ���ԭ�ϣ���ͼ������ij�γ���±�в��ֳɷֺ�������ͼ���Լ��㣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
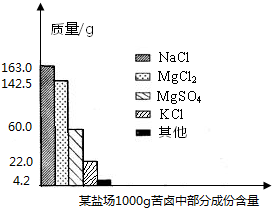 ����Լռ����������71%������ʮ�־�Ŀ���DZ������±�Ǻ�ˮ��ȡʳ�κ�IJ�Һ�����ÿ�±�����Ƶý���þ�Ȼ���ԭ�ϣ���ͼ��ij�γ���±�в��ֳɷֺ�������ͼ���Լ��㣺
����Լռ����������71%������ʮ�־�Ŀ���DZ������±�Ǻ�ˮ��ȡʳ�κ�IJ�Һ�����ÿ�±�����Ƶý���þ�Ȼ���ԭ�ϣ���ͼ��ij�γ���±�в��ֳɷֺ�������ͼ���Լ��㣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com