
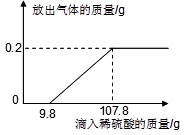
 Cl2��+ H2��+ 2NaOH����ȡһ���������Ȼ�����Һ���е�⣬���Ȼ���ǡ����ȫ��Ӧʱ���õ�51.2 g��Һ������������������ʱ��Ĺ�ϵ��ͼ��ʾ������㣺
Cl2��+ H2��+ 2NaOH����ȡһ���������Ȼ�����Һ���е�⣬���Ȼ���ǡ����ȫ��Ӧʱ���õ�51.2 g��Һ������������������ʱ��Ĺ�ϵ��ͼ��ʾ������㣺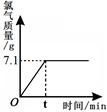
 Cl2��+ H2��+ 2NaOH
Cl2��+ H2��+ 2NaOH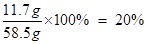
 ��100%���Ȼ��Ƶ������ɸ���������������������������غ㶨�ɣ��Ȼ�����Һ������=����������+�������������ɸ������������������+��Ӧ������������Һ��������
��100%���Ȼ��Ƶ������ɸ���������������������������غ㶨�ɣ��Ȼ�����Һ������=����������+�������������ɸ������������������+��Ӧ������������Һ�������� Cl2��+H2��+2NaOH
Cl2��+H2��+2NaOH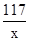 =
=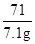
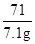 =
=
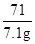 =
=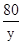
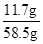 ��100%=20%
��100%=20%


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
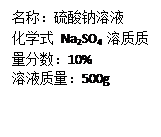
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
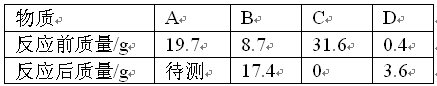
| A��Cһ���ǻ����D�����ǵ��� |
| B����Ӧ�����У�B��D�仯��������Ϊ87��36 |
| C����Ӧ���ܱ�������A������Ϊ19.7 g |
| D����Ӧ��A��C�Ļ�ѧ������֮��Ϊ1��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������

 Na2SO4 + H2O + CO2����
Na2SO4 + H2O + CO2�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
 1g/cm3����Ȼ�������صμ�7.4%�ij���ʯ��ˮ������ȥ100�˳���ʯ��ˮʱ��̼����ǡ�÷�Ӧ��ȫ��
1g/cm3����Ȼ�������صμ�7.4%�ij���ʯ��ˮ������ȥ100�˳���ʯ��ˮʱ��̼����ǡ�÷�Ӧ��ȫ�� ������ȷ��0.01����
������ȷ��0.01�����鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com