
���� ��1������ʯ�ͷ���IJ�����������
��2����Ȼ������Ҫ�ɷ��Ǽ��飬�����ڵ�ȼ������ȼ�����ɶ�����̼��ˮ��д����Ӧ�Ļ�ѧ����ʽ���ɣ�
��3��������Դ��������з���������Դ��ָ����Ⱦ�����Գ������õ���Դ������̫���ܡ����ܡ����ܡ������ܡ���ϫ�ܵȣ�
��� �⣺��1��ʯ�;�������ɵõ����͡����͡�ú�͵ȶ��ֲ�Ʒ�����ʯ�ͣ�
��2����Ȼ������Ҫ�ɷ��Ǽ��飬�����ڵ�ȼ������ȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪCH4+2O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2+2H2O�����CH4+2O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2+2H2O��
��3�������������úͿ���������Դ��̫���ܡ����ܡ����ܡ������ܡ���ϫ�ܡ��������ܵȣ�ȼ�ջ�ʯȼ�Ϸ������ڳ�����Դ��ʹ�ã����AC��
���� �����ѶȲ���ʯȼ��������Դ���п����ȵ㣬�˽ⳣ���Ļ�ʯȼ��������Դ���롢��������ȷ�����Ĺؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| ���� | ���� | ������Ӧѡ�õ��Լ��Ͳ������� | |
| A | N2 | O2 | ��˿����ȼ |
| B | CaO | CaCO3 | �������� |
| C | KNO3��Һ | KOH��Һ | ��������CuSO4��Һ������ |
| D | FeSO4��Һ | CuSO4��Һ | �ӹ��������ۣ����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
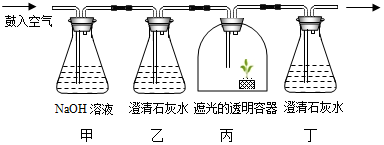
| A�� | ��ʵ������ڼ���ֲ��������ò���CO2 | |
| B�� | ��װ�ñ����ڹ⣬��װ�ö���������������ȼ | |
| C�� | װ�ñ��е�ֲ�ﻻ���ȷ������ӣ�װ�ö��в�����ֻ������� | |
| D�� | װ�ü����������չ�������е�CO2�������ʵ����ɸ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �谱�������л��߷��ӻ����� | |
| B�� | �谱����̼Ԫ������Ԫ�ص���������6��1 | |
| C�� | �谱������Ԫ�ص������������ | |
| D�� | һ���谱������к���һ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������ͬ | B�� | ��������ͬ | ||
| C�� | ��������ͬ | D�� | ������������ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
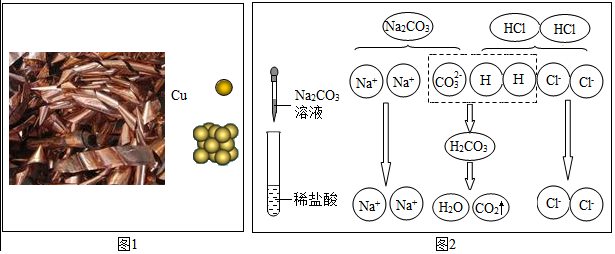
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com