�⣺��1��NH
3+CO
2+H
2O=NH
4HCO
3���ص��ǡ����һ�������ϻ��Ϸ�Ӧ�Ķ�����ص㣬Ϊ���Ϸ�Ӧ��
��2���ٽ���ʹ�����ƴӴ���ˮ��������˵�������Ƶ��ܽ�����¶ȵĽ��Ͷ���С�������¶ȱ仯Ӱ������ԣ�
�ڼ����ɽ������Ӻ����������ӹ��ɵģ�����Ԫ�صļ�Ϊ�������ƣ���ѧʽΪ��Ca��OH��
2���������ơ��Ȼ��ơ��Ȼ�þ�������������������У�ֻ���Ȼ�þ�����������ܷ�����Ӧ����������þ�������Ȼ��ƣ�����ʽΪ��Ca��OH��
2+MgCl
2�TMa��OH��
2��+CaCl
2��
�ۡ�����II�������У�����B�ε������dz�ȥ�������������ƺ��Ȼ��Ƶģ���ͨ����������ȥ��Ϊ�˱�֤�����������ʣ�Ӧ����̼���ƣ��йط�Ӧ����ʽΪ��CaCl
2+Na
2CO
3�T2NaCl+CaCO
3����Ca��OH��
2+Na
2CO
3�T2NaOH+CaCO
3����
�ܼ�����Һ��B���Ƿ������ʵ���Ͼ��Ǽ���̼���ƵĴ��ڣ�����Ϊ����һ��ȡ������II�����˺����Һ�������Թ��У������Ȼ�����Һ���۲죬�������ɫ��������̼�����ѹ�������������ȡ������II�����˺����Һ�������Թ��У�����ϡ���ᣬ�۲죬��������ݣ���̼�����ѹ�����
��ʳ����NH
4HCO
3��Ӧ�Ǹ��ֽⷴӦ�����ݷ�Ӧ�ص㡰���������۲��䡱�жϣ��������Ȼ�狀�̼�����ƣ�̼�����Ƶ��ܽ�Ƚ�С���ᾧ����������Ϊ���������Է���ʽΪ��NH
4HCO
3+NaCl=NaHCO
3��+NH
4Cl��
��4���⣺���ɶ�����̼������Ϊ100g+5.6g-103.4g=2.2g
����Ʒ��̼���Ƶ�����Ϊx
Na
2CO
3+2HCl�T2NaCl+CO
2��+H
2O
106 44
x 2.2g
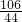
=
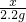
x=5.3g
��ô�����Ʒ��̼���Ƶ���������Ϊ
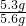
��100%=94.6%
��������Ʒ��̼���Ƶ���������Ϊ94.6%��
�ʴ�Ϊ����1�����ϣ�
��2���ٴ�
��Ca��OH��
2��Ca��OH��
2+MgCl
2�TMa��OH��
2��+CaCl
2��
��CaCl
2+Na
2CO
3�T2NaCl+CaCO
3����Ca��OH��
2+Na
2CO
3�T2NaOH+CaCO
3����
�ܷ���һ��ȡ������II�����˺����Һ�������Թ��У������Ȼ�����Һ���۲죬�������ɫ��������̼�����ѹ�����
��������ȡ������II�����˺����Һ�������Թ��У�����ϡ���ᣬ�۲죬��������ݣ���̼�����ѹ�����������������Ҳ�ɣ�
��NH
4HCO
3+NaCl=NaHCO
3��+NH
4Cl��
��4���⣺���ɶ�����̼������Ϊ100g+5.6g-103.4g=2.2g
����Ʒ��̼���Ƶ�����Ϊx
Na
2CO
3+2HCl�T2NaCl+CO
2��+H
2O
106 44
x 2.2g
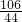
=
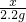
x=5.3g
��ô�����Ʒ��̼���Ƶ���������Ϊ
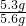
��100%=94.6%
��������Ʒ��̼���Ƶ���������Ϊ94.6%��
��������1�����ݻ��Ϸ�Ӧ�Ķ�����ص�ش�
��2���ٸ��������Ƶ��ܽ�����¶�Ӱ�������ش�
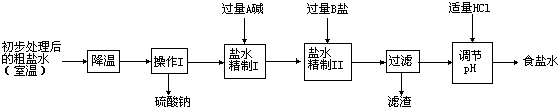
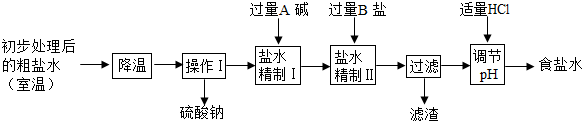
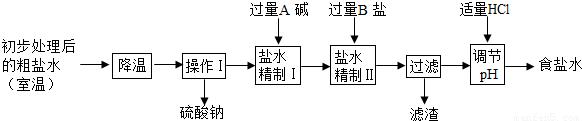
�ڸ��ݼ����ɻش�ǰһ�գ����ݡ�����I���������漰�����������ơ��Ȼ��ơ��Ȼ�þ���������Ƶ����ʻش����ķ�Ӧ��
�۸���̼���ơ��������ƺ��Ȼ��ƵĻ�ѧ���ʼ����ӵ�ԭ��ش�
�ܸ���̼���ƵĻ�ѧ���ʺͼ��鷽���ش�
�ݸ��ݵڶ���ʳ����NH
4HCO
3��Ӧ�Ǹ��ֽⷴӦ���Լ�̼�����Ƶ��ܽ�Ƚ�С����Ϣ��д����ʽ��
��4�����ⷢ���ķ�ӦΪNa
2CO
3+2HCl�T2NaCl+CO
2��+H
2O�����������غ㶨�ɼ������Ӧ���ɵĶ�����̼���������ٸ��ݶ�����̼�������ͻ�ѧ����ʽ���㴿����Ʒ��̼���Ƶ������������ô�����Ʒ��̼���Ƶ�����������
������ѧ��Դ������������ַ�����������������ҵ�dz��л�ѧ��Ҫ��Ӧ��֮һ���ǿ����ص㣬���漰��ѧ����ʽ����д��������̼�Ͱ���ͨ���˳��ԭ��Ӧʱ����̼�����Ƶ�ԭ���������ܽ�ȵĹ�ϵ���ص����ݣ�


 Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2��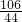 =
=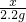
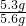 ��100%=94.6%
��100%=94.6%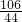 =
=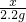
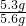 ��100%=94.6%
��100%=94.6%





 Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2��