19��ij��Ȼ��Ȫˮ����Ҫ�ɷ����£��������Ķ�����գ�
��Ҫ�ɷ֣�mg/L����
̼�������HCO3-����173-205 �����ӣ�Cl-����1.0-8.0 �������SO42-����16.08
-19.52 �����ӣ�Na+����8-50 PHֵ��7.8��0.5��1����SO42-�������֡�2���ĺ������������������λ����� ��
��2���ÿ�Ȫˮ������ �ԣ���ᡱ��������С�����
��3���ճ�������������ˮ ������ˮ��Ӳˮ����ͨ��������� ��������ˮ��Ӳ�ȣ�
�������⣺��1�����ݻ�ѧ������Χ���ֵ�����������ɣ��������Ͻǵ����ִ����Ƹ����������ĵ�ɣ�
��2��������Һ�������PHֵ�Ĺ�ϵ�������ɣ���PH��7����Һ�Լ��ԣ���PH=7����Һ�����ԣ���PH��7����Һ�����ԣ�
��3������Ӳˮ����ˮ�ĸ�����ߵ������������ɣ�Ӳˮ�к��н϶�Ca2+��Mg2+����ˮ�к���Ca2+��Mg2+���ٻ���
����������Ca2+��Mg2+��Ӧ�����ɰ�ɫ���������ֳ������Ǹ�������ʽ��
����Ӳˮʱ�������з�Ӧ��
Ca��HCO3��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCO3��+H2O+CO2��
Mg��HCO3��2$\frac{\underline{\;\;��\;\;}}{\;}$MgCO3��+H2O+CO2��
MgCO3+H2O$\frac{\underline{\;\;��\;\;}}{\;}$Mg��OH��2��+CO2��
����ʱCa2+��Mg2+����������ˮ��Ӳ�Ƚ��ͣ� ����⣺��1������SO42-���е�����2��ʾ�������2����λ����ɣ�
�ʴ�Ϊ���������2����λ�����
��2������������Ϣ���ÿ�Ȫˮ��PHΪ7.8�����Լ��ԣ��ʴ�Ϊ����
��3��Ӳˮ�м������ˮ������ֱ�ɫ����������ˮ�м������ˮ������ִ��������Կ����÷���ˮ������Ӳˮ����ˮ��
�������Ӳˮʱ��Ca2+��Mg2+������CaCO3��Mg��OH��2����ʽ�������������Լ�����еķ������Խ���ˮ��Ӳ�ȣ�
�ʴ�Ϊ������ˮ�� ������� ��������ѧ������Χ���ֵ������dz����⣬�����۳���ͬѧ��˵Ҳ���ѵ㣬���ڸ���Ҿټ������ӿ�����
2Mg��ʾ2��þԭ�� Mg2+��ʾþ���� $\stackrel{+2}{Mg}$��ʾþ����2��
��ϰ��ϵ�д�
���ϰ��
��Ŀ�����л�ѧ
��Դ��
���ͣ�
25��ij��Ȼ��Ȫˮ����Ҫ�ɷ����£��������Ķ�����գ�  ��1����SO 42-�������֡�2���ĺ�����
��2����λ����ɵ���������� �� ��2���ÿ�Ȫˮ��
�� �ԣ���ᡱ��������С����� ��3���ճ���������
����ˮ ������ˮ��Ӳˮ����ͨ��
������� ��������ˮ��Ӳ�ȣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ
��Դ��
���ͣ��Ķ�����
11��ij��Ȼ��Ȫˮ����Ҫ�ɷ����£��������Ķ�����գ�
��Ҫ�ɷ֣�mg/L����
̼�������HCO3-����173-205 �����ӣ�Cl-����1.0-8.0
�������SO42-����16.08-19.52 �����ӣ�Na+����8-50
þ���ӣ�2.5-12.5 PHֵ��7.8��0.5��1��þ���ӻ�ѧ���ſɱ�ʾΪMg2+ ����SO42-�������֡�2���ĺ�����һ����������Ӵ�������λ�ĸ���� ��
��2���ÿ�Ȫˮ���� �ԣ���ᡱ��������С�����
��3���ճ�������������ˮ ������ˮ��Ӳˮ����ͨ����� ��������ˮ��Ӳ�ȣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ
��Դ��
���ͣ�
 ��1����ͼ��ת������β�����к��������ʾ��ͼ�� �ٷ�Ӧ���ͼʾ�к��� 3 3 �ַ��ӣ� ��ͼ����ʾ�Ļ�ѧ����ʽ �� �۴�ͼ���㻹�ܻ�ȡ����Ϣ�� �ڻ�ѧ�仯�з��ӿ����ٷ֣���ѧ��Ӧǰ��Ԫ�ص�����䣬�������ԭ�ӹ��ɣ��������� �ڻ�ѧ�仯�з��ӿ����ٷ֣���ѧ��Ӧǰ��Ԫ�ص�����䣬�������ԭ�ӹ��ɣ��������� ������һ�֣� ��2��ij��Ȼ��Ȫˮ����Ҫ�ɷ����±����������Ķ�����գ�
| ��Ҫ�ɷ֣�mg/L����̼�������HCO3-����173-205 �����ӣ�1.0-8.0 ��������ӣ�16.08-19.52 �����ӣ�Na+����8-50 þ����Mg2+��2.5-12.5 pHֵ��7.8��0.5����������ӵķ���Ϊ SO42- SO42- �������ӵķ���ΪCl- Cl-
���ճ������г�������ˮ ����ˮ ������ˮ��Ӳˮ��
��ũҵ���ֽ���ֲ��ʱ������ˮ�����Ϊ��ࡢ�ι��Ŀ������Լ��ˮ ��Լ��ˮ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ
��Դ��
���ͣ������
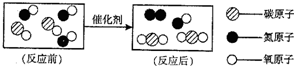 ��1����ͼ��ת������β�����к��������ʾ��ͼ�� ��1����ͼ��ת������β�����к��������ʾ��ͼ��
�ٷ�Ӧ���ͼʾ�к���______�ַ��ӣ�
��ͼ����ʾ�Ļ�ѧ����ʽ______��
�۴�ͼ���㻹�ܻ�ȡ����Ϣ��______������һ�֣�
��2��ij��Ȼ��Ȫˮ����Ҫ�ɷ����±����������Ķ�����գ�
| ��Ҫ�ɷ֣�mg/L����̼�������HCO3-����173-205�������ӣ�1.0-8.0����������ӣ�16.08-19.52�� �����ӣ�Na+����8-50��þ����Mg2+��2.5-12.5�� pHֵ��7.8��0.5 |
����������ӵķ���Ϊ______�������ӵķ���Ϊ______ ���ճ������г���______������ˮ��Ӳˮ�� ��ũҵ���ֽ���ֲ��ʱ������ˮ�����Ϊ��ࡢ�ι��Ŀ����______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ
��Դ��2012��㶫ʡ÷����ѧ�п���ѧģ���Ծ��������棩
���ͣ������
��1����ͼ��ת������β�����к��������ʾ��ͼ�� �ٷ�Ӧ���ͼʾ�к��� �ַ��ӣ� ��ͼ����ʾ�Ļ�ѧ����ʽ �� �۴�ͼ���㻹�ܻ�ȡ����Ϣ�� ������һ�֣� ��2��ij��Ȼ��Ȫˮ����Ҫ�ɷ����±����������Ķ�����գ� | ��Ҫ�ɷ֣�mg/L����̼�������HCO3-����173-205 �����ӣ�1.0-8.0 ��������ӣ�16.08-19.52 �����ӣ�Na+����8-50 þ����Mg2+��2.5-12.5 pHֵ��7.8±0.5 |
����������ӵķ���Ϊ �������ӵķ���Ϊ ���ճ������г��� ������ˮ��Ӳˮ�� ��ũҵ���ֽ���ֲ��ʱ������ˮ�����Ϊ��ࡢ�ι��Ŀ���� �� 
�鿴�𰸺ͽ���>>
| | |



 ����ѧ����ϵ�д�
����ѧ����ϵ�д�
