
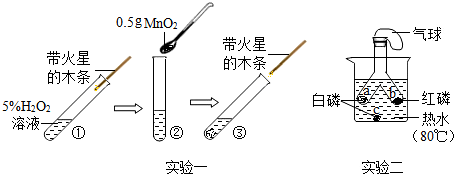
���� ��1�����ݶ������̶Թ�������ķֽ��д����ûش𣻸��ݴ����Ķ�����ص�ش��������ݴ����Ķ�����ص�ش𣻸��ݶԱ�ʵ���̽�������ش�
��2�����ݿ�ȼ���ȼ������������
��� �⣺�������ص��ǡ�һ�䣬�����䡱������Ӧ���ʱ䣬�����ͻ�ѧ���ʲ��䣻
��1��̽�����������Ƿ��ܸı䷴Ӧ�����ʣ�Ҫͨ��������������벻�Ӷ������̵�������жԱȣ����ܵó���ѧ�����Ľ��ۣ�
�������̶Թ�������ķֽ��д����ã��ܼӿ���ֽ������������������ʣ��������������ݣ������ǵ�ľ����ȼ���������ص��ǡ�һ�䣬�����䡱����Ӧǰ�����������䣬���Զ������̵�������Ϊ0.5g���������ص��ǡ�һ�䣬�����䡱�����з�Ӧ���ʸı䣬�����Ǽӿ죬������������Һ�������ݷų��������ǵ�ľ����ȼ��
��2��a���İ����¶ȴﵽ�Ż�㣬������е������Ӵ�������ȼ�յ�������ȼ��һ��ʱ���Ϩ��Ŀ���ԭ�������������꣬�����վ���
�ʴ�Ϊ��
��1�������գ�������Ѹ�ٷų���������ľ����ȼ�� ���ڣ�
��2��a�� BC���д���©���÷֣���
���� �������������dz�����Ҫ��������֮һ���ǿ�����ص���ȵ㣬��Ҫ����Դ����������ø�������⣬�Դ�Ч��Ӱ�����ص�̽���ȣ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ϊ��ʹ���Ƶ���Һ��������������ȷ��������Ͳ���ܽ����� | |
| B�� | ʵ������ȡ������̼ʱ���ȼ��װ�������ԣ��ټ���ҩƷ | |
| C�� | ���ȸ������������������ˮ���ռ���ʵ�����ʱ��Ϩ��ƾ����ٳ����� | |
| D�� | ����������������������ʯ�����ϣ��þƾ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ͻӷ�--���������� | |
| B�� | ���㲻��������--�����ڲ����˶� | |
| C�� | �������д���--���Ӽ��м�� | |
| D�� | �������ֽ�ɹ�������--��ѧ�仯�з��ӿɷ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
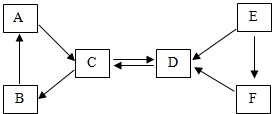 ȡA----F���ֳ������ʣ�����A�����ڸ�������������B�dz��õĹ���������A��B��C���嶼����ͬ�ֽ���Ԫ�أ�E�Ǻ�ɫ���嵥�ʣ�D��E��F������ͬ�ַǽ���Ԫ�أ����ǵ�ת����ϵ��ͼ��ʾ����ش�
ȡA----F���ֳ������ʣ�����A�����ڸ�������������B�dz��õĹ���������A��B��C���嶼����ͬ�ֽ���Ԫ�أ�E�Ǻ�ɫ���嵥�ʣ�D��E��F������ͬ�ַǽ���Ԫ�أ����ǵ�ת����ϵ��ͼ��ʾ����ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com