
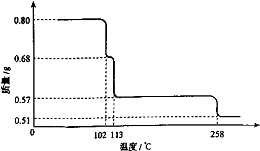
 0.80g CuSO4?5H2O��Ʒ������ˮ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ���ͼ��ʾ��
0.80g CuSO4?5H2O��Ʒ������ˮ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ���ͼ��ʾ��
| ||
| ||
| 250 |
| 18n |
| 0.80g |
| 0.23g |
| ||
| ||


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
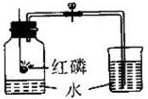 ��ͼ��ʾ�Dzⶨ����������������ʵ��װ�ã�
��ͼ��ʾ�Dzⶨ����������������ʵ��װ�ã�| 1 |
| 5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
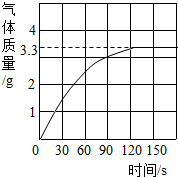 ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽12.5gˮ���У�����CO2����������ͼ��ʾ����ʾ����Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2�� Mg��OH��2+2HCl�TMgCl2+2H2O
ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽12.5gˮ���У�����CO2����������ͼ��ʾ����ʾ����Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2�� Mg��OH��2+2HCl�TMgCl2+2H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
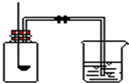 �ú����������������������IJⶨʵ�飬�ش��������⣮
�ú����������������������IJⶨʵ�飬�ش��������⣮| 1 |
| 5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
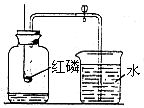 ��ͼ�Dzⶨ����������������ʵ��װ�ã������ʵ��ش����⣺
��ͼ�Dzⶨ����������������ʵ��װ�ã������ʵ��ش����⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��ʾijЩ���ʼ�ת����ϵ����Ӧ�����Ͳ��ֲ�����ʡ�ԣ�������A��EΪ�����������AΪ��ɫ��ĩ��B��D������ͬԪ����ɵ���ɫҺ�壬��B��������ɱ�����ã�C��Y��ZΪ��ɫ���壬����Y�ж���X������Ľ�������ش��������⣺
��ͼ��ʾijЩ���ʼ�ת����ϵ����Ӧ�����Ͳ��ֲ�����ʡ�ԣ�������A��EΪ�����������AΪ��ɫ��ĩ��B��D������ͬԪ����ɵ���ɫҺ�壬��B��������ɱ�����ã�C��Y��ZΪ��ɫ���壬����Y�ж���X������Ľ�������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
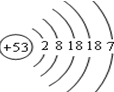
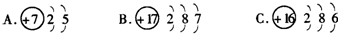
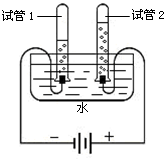
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com