��2012?�ֶ�������ģ��2011��9�£���¶�����౻�غ����°���������������������ߵĽ��ǣ�����ͬѧ�ǿ�չ�˶�����ɷּ����õ�̽���������������ϵ�֪��
��1�����������ֹ㷺����ϴ�Ӽ���ҽ�������Ŀ�������������������������ţ����ר���ѱ�ʾ��Ŀǰ��û�п�ѧ�о�����֤�������е����������°���ʹ��Ũ�Ȳ�����0.3%���������ǰ�ȫ�ģ�������ʶȱ����ѧ�Ե���
AB
AB
��
A��ֹͣ�����ʹ�ø�¶���Ʒ
B��ʹ���˻�ѧ���Ӽ���ʳƷ�����嶼��Σ��
C�����ʶ������Ӱ�����������йأ����������ʱ�
D����������г��ֵ��йػ�ѧ���¼�������Ҫ�������Է�����������ȷ�ж�
��2���ܶ�Ʒ��������С����������������еġ�������ָ
B
B
��
A�������� B����Ԫ�� C����ԭ��
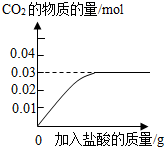
��3������ȥ����Ҫ��������Ħ�����ã�ijƷ�������е�Ħ������̼��ƣ�Ϊ�˼��鲢�ⶨ������̼��Ƶ�����������ͬѧ��ȡ��10g���࣬��������ϡ���Ტ���裮ʵ������м�¼�����������γ���ͼ���ߣ�
�ټ���10g������̼��Ƶ����ʵ���
0.03mol
0.03mol
�������ݻ�ѧ����ʽ��ʽ���㣩��
�ڸ�Ʒ��������̼��Ƶ���������Ϊ
30%
30%
��
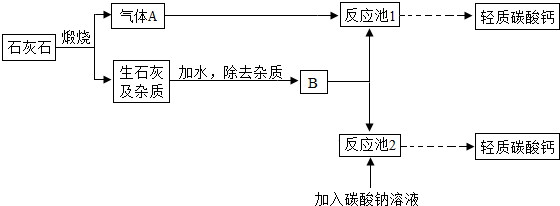
��4�������е�����̼��ƿ���ʯ��ʯ���Ʊ���ij��ѧ��ȤС�����������ת�����̣�
��֪��CaCO
3+CO
2+H
2O��Ca��HCO
3��
2��Ca��HCO
3��
2 CaCO
3��+CO
2��+H
2O
������A�Ļ�ѧʽΪ
CO2
CO2
��д����Ӧ��1�з�����Ӧ�Ļ�ѧ����ʽ
Ca��OH��2+CO2�TCaCO3��+H2O��
Ca��OH��2+CO2�TCaCO3��+H2O��
��
��Ϊ���������Ч�ʣ���ȡ����̼���ʱ��B���Ϊ
ʯ����
ʯ����
��ѡ�ʯ���顱����ʯ��ˮ������������Aͨ�뷴Ӧ��1��ʱ����öԳ������ʽ����ȣ�Ŀ���Ƿ�ֹ����
Ca��HCO3��2
Ca��HCO3��2
��д��ѧʽ����
�۽������ת�����̣��Աȷ�Ӧ��1�ͷ�Ӧ��2��ȡ̼��Ƶķ�����ǰ�߸�����������ɫ��ѧ�����������
�������CO2
�������CO2
��





