
| ��� | ���� | NaOH��Һ | ��t/�� |
| 1 | 3.65% | 2.00% | 3.5 |
| 2 | 3.65% | 4.00% | x |
| 3 | 7.30% | 8.00% | 14 |
| �� �� �� Ŀ | �� �� ʱ �� | ������g�� |
| ���� | 10.00 | |
| װ��+ϡ�������� | 241.20 | |
| װ��+ϡ��������+���� | ��Ӧ��ʼ��15�� | 249.20 |
| װ��+ϡ��������+���� | ��Ӧ��ʼ��35�� | 249.00 |
| װ��+ϡ��������+���� | ��Ӧ��ʼ��55�� | 249.00 |
 �����Ƴ�x=7��
�����Ƴ�x=7�� =
=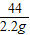
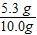 ×100%=53%
×100%=53%

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��� | ���� | NaOH��Һ | ��t/�� |
| 1 | 3.65% | 2.00% | 3.5 |
| 2 | 3.65% | 4.00% | x |
| 3 | 7.30% | 8.00% | 14 |
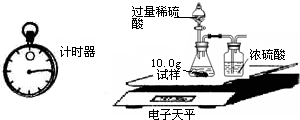
| �� �� �� Ŀ | �� �� ʱ �� | ������g�� |
| ���� | 10.00 | |
| װ��+ϡ�������� | 241.20 | |
| װ��+ϡ��������+���� | ��Ӧ��ʼ��15�� | 249.20 |
| װ��+ϡ��������+���� | ��Ӧ��ʼ��35�� | 249.00 |
| װ��+ϡ��������+���� | ��Ӧ��ʼ��55�� | 249.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
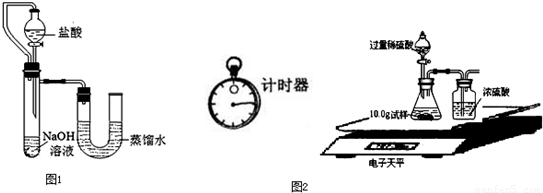
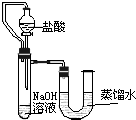
 ����֮�䷢����ѧ��Ӧʱ�������������Ե�������Щ��ѧ��Ӧȴ�۲첻�����Ե�����ij��ȤС��ͬѧΪ֤��NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ���Ӳ�ͬ�Ƕ����������ʵ�鷽����������ʵ�飮
����֮�䷢����ѧ��Ӧʱ�������������Ե�������Щ��ѧ��Ӧȴ�۲첻�����Ե�����ij��ȤС��ͬѧΪ֤��NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ���Ӳ�ͬ�Ƕ����������ʵ�鷽����������ʵ�飮| ��� | ���� | NaOH��Һ | ��t�� |
| 1 | 3.65% | 2.00% | 3.5 |
| 2 | 3.65% | 4.00% | |
| 3 | 7.30% | 8.00% | 14 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com