
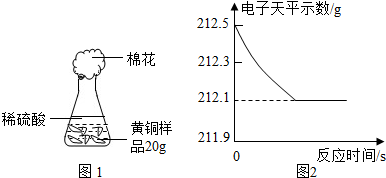
| ��Ʒ��ͭ������ |
| ҩƷ���� |
| 65 |
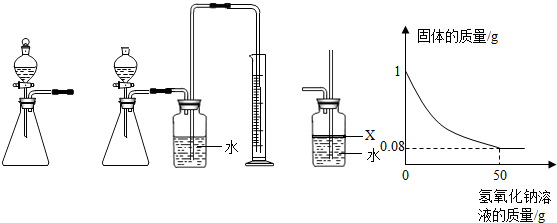
| X |
| 2 |
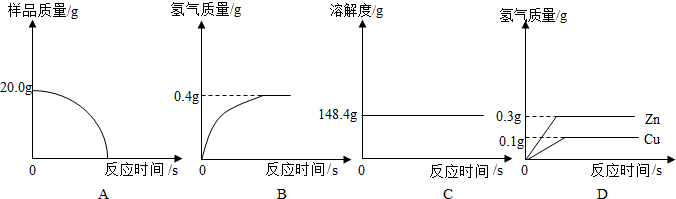
| 0.4g |
| 161 |
| y |
| 2 |
| 0.4g |
| (20g-13g) |
| 20g |
| 32.2g |
| 212.1g-44.1g-7g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2013?��ͨһģ������۳�Ϊ������ϲ����Ȼ��һЩ���������ñ��Ƿ�ð����������ۣ�һ�о���ѧϰС��Ϊ�˼���ij����۵���٣�����������̽����
��2013?��ͨһģ������۳�Ϊ������ϲ����Ȼ��һЩ���������ñ��Ƿ�ð����������ۣ�һ�о���ѧϰС��Ϊ�˼���ij����۵���٣�����������̽�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��Ӧʱ��/�� | 0 | 2 | 4 | 6 | 8 | 10 |
| �ձ�����ʢ��������/�� | 80.0 | 79.0 | 78.3 | 77.9 | 77.8 | 77.8 |
| �ṩ��ҩƷ | ���������� |
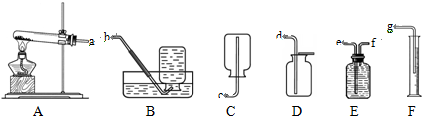
| a������ b��ϡ���� |
�ٷ�Ӧǰ���۵����� �ڷ�Ӧ�����ۺ��������� ��AgCl������ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����۳�Ϊ������ϲ����Ȼ��һЩ���������ñ��Ƿ�ð����������ۣ�һ�о���ѧϰС��Ϊ�˼���ij����۵���٣�����������̽����
����۳�Ϊ������ϲ����Ȼ��һЩ���������ñ��Ƿ�ð����������ۣ�һ�о���ѧϰС��Ϊ�˼���ij����۵���٣�����������̽�����鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com