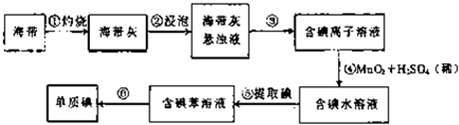
ij�о���ѧϰС����Ʋ�����������ʵ�飮
��1������ͬѧ����ͼװ����ȡ���﴿���Ķ�����̼���壮��ش��������⣺
������X��������
����©��
����©��
��ʵ��ǰ���رջ�������X�м���ˮʹX��Һ������Թ��е�Һ�棬һ��ʱ���Һ�汣�ֲ���˵��
Aװ�õ�����������
Aװ�õ�����������
��
��Ϊ��ȥCO
2�л��е�HCl���壬Bװ����ʢ�ű���NaHCO
3��Һ��д���÷�Ӧ�Ļ�ѧ����ʽ
NaHCO3+HCl=NaCl+CO2��+H2O
NaHCO3+HCl=NaCl+CO2��+H2O
��
��Ҫ�õ������CO
2��Cװ��Ӧʢ��
Ũ����
Ũ����
�����Լ����ƣ���
����˵��װ��B��C���Լ����ܽ�����ԭ��
��������������ͨ������NaHCO3��Һ��������ˮ��������
��������������ͨ������NaHCO3��Һ��������ˮ��������
��
��2������ͬѧΧ��CO��ԭCuO�����ĺ�ɫ�����Ƿ�һ����Cu����̽����
���������ϡ�
��Cu
2O��ĩ�ʺ�ɫ����������Һ�У�Cu
2O+H
2SO
4=Cu+CuSO
4+H
2O
���ڿ����и�������ʱ��Cu
2O�ȶ�����CuO���ֽ�����Cu
2O��O
2��������롿
�����ɫ������Cu�� �����ɫ������Cu
2O�� �����ɫ������
Cu2O��Cu�Ļ����
Cu2O��Cu�Ļ����
��
��ʵ��̽����
��������Cu����Cu
2O�Ļ�ѧ����ʽ��
��
��������ݷ���
��1��ȡһ�������ĺ�ɫ����a g���ڿ����и������գ�ֱ���������ٷ����ı䣬�������ù�������Ϊb g��
����a=b�������
II
II
������
����b��a����˵����ɫ������һ������
Cu
Cu
���ʣ�
��2����ȡ������ɫ����Ͷ��ϡ�����У���ַ�Ӧ����Һ����ɫ�����ݴ�����˵���˺�ɫ�����к���
Cu2O
Cu2O
������������1.6g��ԭ���������ϡ���ᷢ����Ӧ��������
2.88
2.88
g��

 ij�о���ѧϰС����Ʋ�����������ʵ�飮
ij�о���ѧϰС����Ʋ�����������ʵ�飮


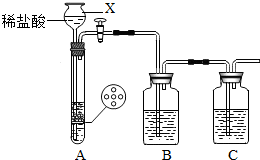 ��2012?�紨��һģ��ij�о���ѧϰС����Ʋ�����������ʵ�飮
��2012?�紨��һģ��ij�о���ѧϰС����Ʋ�����������ʵ�飮
 ij�о���ѧϰС����Ƶ�ʵ��װ�ã���ͼ�����ȿ���ȡ���壬�ֿ�������֤�������ʣ�
ij�о���ѧϰС����Ƶ�ʵ��װ�ã���ͼ�����ȿ���ȡ���壬�ֿ�������֤�������ʣ�