Ϊ��ȷ��ij���ȼ���������������������ɣ��ֱ��������ʵ�飮��֪�����������ڸ����·�Ӧ���������������������������Ƶ�ˮ��Һ��Ӧ����ƫ�����ƣ�NaAlO2����������
��1����ȡag��Ʒ�������м�������������������Һ��������ɵ�����Ϊbg����Ӧ�Ļ�ѧ����ʽΪ______����Ʒ����������Ϊ______g��
��2����ȡag��Ʒ��ʹ���ڸ�����ǡ����ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ______����Ʒ��������������������Ϊ______��
��3������2���з�Ӧ������ȴ�������м������������ᣬ������ɵ���������Ϊcg����������루1�������õ������������c��b=______��
�⣺��1������Ҫ����������x
2Al+2NaOH+2H
2O=2NaAlO
2+3H
2��
54 6
x bg
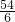
=
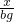
x=9bg
�ʴ�Ϊ��9b
��2����ѧ��Ӧ�����ʵ������ȵ�����Է��������͵ı�
2Al+Fe
2O
3
2Fe+Al
2O
354 160
��Ʒ��������������������Ϊ80��27
�ʴ�Ϊ��80��27
��3����Ϊ������Ʒ��Ϊa g���ɣ�1������2������Ʒ�е�Al�����ɵ�Fe�����ʵ���֮��Ϊ1��1����� Fe+2HCl=FeCl
2+H
2�����������������ʵ�����2mol����1������3mol������������Ӧ���ɵ��������ʵ����ǣ�2mol���ɵ�c��b=2��3��
�ʴ�Ϊ��2��3
��������1�����ݻ�ѧ����ʽ�ļ��㣺�������ɵ�����������������������
��2�����û�ѧ����ʽ�����ʵ�����֮�ȵ�����Է��������͵ıȽ����
��3����Ϊ��1����2����ԭ����ﶼ��ag�����У�2���ķ�Ӧ�п��Կ�����������⟵����ʵ�����ȣ��ٽ����������ķ�Ӧ�ɶ����������������Ƚ��з������ɣ�
�����������Ƕ����ʷ�Ӧʱ�������Ŀ��飬�ص��ǶԻ�ѧ����ʽ�ļ���Ȼ���֪ʶ�Ŀ��飬�ѵ����ڵ��������ʵ����Ĺ�ϵ���Ƶ���
��Ŀ�����л�ѧ
��Դ��2007�꽭��ʡ��ͨ�к�����ʵ��ѧУ���꼶��ѧ����ѡ�����Ծ��������棩
���ͣ������
Ϊ��ȷ��ij���ȼ���������������������ɣ��ֱ��������ʵ�飮��֪�����������ڸ����·�Ӧ���������������������������Ƶ�ˮ��Һ��Ӧ����ƫ�����ƣ�NaAlO2����������
��1����ȡag��Ʒ�������м�������������������Һ��������ɵ�����Ϊbg����Ӧ�Ļ�ѧ����ʽΪ ����Ʒ����������Ϊ g��
��2����ȡag��Ʒ��ʹ���ڸ�����ǡ����ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ ����Ʒ��������������������Ϊ ��
��3������2���з�Ӧ������ȴ�������м������������ᣬ������ɵ���������Ϊcg����������루1�������õ������������c��b= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ
��Դ��2007�긣��ʡȪ���н������ӽ���ѧ���꼶��ѧ����ѡ�����Ծ��������棩
���ͣ������
Ϊ��ȷ��ij���ȼ���������������������ɣ��ֱ��������ʵ�飮��֪�����������ڸ����·�Ӧ���������������������������Ƶ�ˮ��Һ��Ӧ����ƫ�����ƣ�NaAlO2����������
��1����ȡag��Ʒ�������м�������������������Һ��������ɵ�����Ϊbg����Ӧ�Ļ�ѧ����ʽΪ ����Ʒ����������Ϊ g��
��2����ȡag��Ʒ��ʹ���ڸ�����ǡ����ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ ����Ʒ��������������������Ϊ ��
��3������2���з�Ӧ������ȴ�������м������������ᣬ������ɵ���������Ϊcg����������루1�������õ������������c��b= ��
�鿴�𰸺ͽ���>>

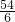 =
=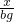
 2Fe+Al2O3
2Fe+Al2O3

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�