
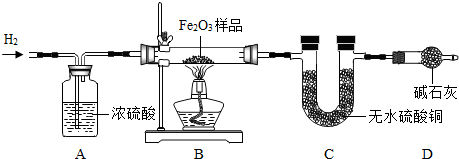
���� ��1���������п�ȼ�ԣ�Ϊ��ֹ��ը������ǰӦ����ͬ�������ž��Թ��ڵĿ�����
��2��C�е���ˮ����ͭ����ˮ�����ɣ�
��3����ʡȥDװ�ã�C���������������ý�����
��4��C�����ӵ�����������ˮ���������ɴ˿������Ԫ���������������Fe2O3������������������
��� �⣺��1����Ϊ�������п�ȼ�ԣ�װ�����п���ʱ�ᷢ����ը���ʴ�Ϊ����ȥװ���еĿ�������ֹ����ʱ������ը��
��2����ˮ����ͭ��ˮ�������ʴ�Ϊ����ɫ��ĩ������
��3�����û��Dװ�ã������е�ˮ���������U�ιܣ�����ˮ����ͭ�����գ�����ˮ�����������ʹ��õ���Ʒ��Fe2O3������������ʵ�ʵ�ƫ�ʴ�Ϊ��ƫ��4����ΪC�����ӵ�����������ˮ������2.7��������Ԫ�ص�������2.7��$\frac{16}{18}$=2.4g��Fe2O3������2.4g��$\frac{48}{160}$=8g������Ʒ��Fe2O3����������Ϊ$\frac{8g}{10g}$��100%=80%���ʴ�Ϊ��80%��
���� ����ͨ��һ����̼��ԭ��������ʵ�鿼����Ʒ���������������������������輰�������������ۺ�̽���⣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
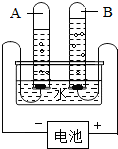 ������ͼ��ʾ�����й��ڵ��ˮʵ���������ȷ���ǣ�������
������ͼ��ʾ�����й��ڵ��ˮʵ���������ȷ���ǣ�������| A�� | �������������������2��1 | B�� | A�Թ�������ʹ�����ǵ�ľ����ȼ | ||
| C�� | ˮ�����⡢������Ԫ����ɵ� | D�� | B�Թ����ƽ����淢�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������ͨ���߲���Я���ƾ�����ȼ��Ʒ | |
| B�� | ����ʱ���Ż��ù��Ǹ��� | |
| C�� | ����ʧ��ʱ�������������Ŵ���������Ũ�̴��Ŵ��ų� | |
| D�� | ��Χ���ڻ�����ʱ����ʪë����ס�ڱǵ����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
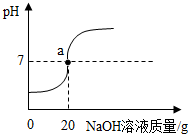 ��50g�Ȼ�����Ȼ��ƵĻ����Һ�еμ�������������Ϊ20%������������Һ���õ���Һ��pH�仯������ͼ��ʾ����ش��������⣺
��50g�Ȼ�����Ȼ��ƵĻ����Һ�еμ�������������Ϊ20%������������Һ���õ���Һ��pH�仯������ͼ��ʾ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
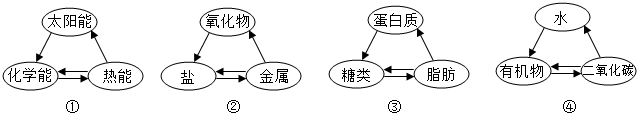
| A�� | �٢ڢ� | B�� | �ڢ� | C�� | �ڢۢ� | D�� | �ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����B��
����B�� ����Ӧ����C��
����Ӧ����C�� ������Ӧǰ����Ӽ�����Ŀ�ı仯����÷�Ӧ�Ļ�ѧ����ʽ�У���ѧ������֮��Ϊ��������
������Ӧǰ����Ӽ�����Ŀ�ı仯����÷�Ӧ�Ļ�ѧ����ʽ�У���ѧ������֮��Ϊ��������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com